Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
Biomanufacturing automation is an established mission-critical step in the commercialization pathway for conventional therapeutics, including small molecules and monoclonal antibodies (MAbs) (1). The prospect of a potential biologic progressing into late-stage clinical trials without a robust biomanufacturing strategy to support at least pilot-plant scale bioprocessing is simply unthinkable. Conversely, the cell therapy industry (or at least a significant proportion of it) regard this as a trend that is unlikely to be mirrored as the industry develops.
The aim of this piece is to assess the risks and benefits of the automation of cell therapy biomanufacturing by focusing in particular on the feasibility of process automation and its impact on regulatory compliance and return on investment. Interestingly, the authorship — composed of academia and industry — spans the product development pathway. One author will make the case that in some respects it is challenging to translate lab-scale processes into automated large-scale manufacturing strategies and advocate delaying automation and any costs incurred until later in the development cycle. Another — a leading supplier of automated biomanufacturing technologies, with significant experience automating cell therapy bioprocesses — will justify a strong belief that cell therapy process automation is a critical and wholly achievable product development goal, best addressed as early as possible in the translation pathway. Finally, an author positioned at the interface between academic biomedical research and finance will evaluate the prospect of biomanufacturing automation from the viewpoint of potential investors, eager to ensure that value is created and protected as efficiently as possible. It should be an interesting tussle.

Feasibility: Barriers to Cell Therapy Automation
Manual bioprocessing techniques (notably, cell culture) are critically important in terms of skill development for employees in industry and students within academia. Such skills provide a foundation for navigating future automation and operation strategies such as cell characterization studies (2). Further, in addition to capital investment costs, the management of automated biomanufacturing equipment in academic settings can be challenging. For example, access to resources must be shared amongst multiple parties with different work schedules, funding structures, and process materials.
In some situations (e.g., autologous cell therapy production), manufacturing limitations render automation extremely challenging, in part due to inherent interdonor source material variability. For example, populations of bone-marrow–derived mesenchymal stromal cells (BM-MSCs) from different individuals (where the start population of cells comprises a heterogeneous mix of subclones) will age at different rates and have variable replicative lifespans. This is due largely to intrinsic telomere dynamics, difference in donor ages, oxidative stress status, and stochastic events (3, 4). A single defined set of automated process parameters for cell expansion will not discriminate among individual samples. Consequently, products will exhibit different source material characteristics after amplification of the start population.
Evaluating Automation Success
Evaluating your automation strategy success requires that you assess
the extent of process automation
the extent to which automation enables and captures a market
Further, the start material will exhibit some variability if it is in the form of a tissue biopsy obtained by a clinician. This is a consequence of the physical nature of the tissue being subject to variability between donor sites in terms of accessibility, tissue quality, surgical/sampling accuracy, and the exact quantity of tissue removed (5). Therefore, skilled technicians will isolate and plate cells from tissue biopsies and conduct morphological assessments to evaluate the likely risks and benefits arising from processing a particular patient sample.
Any suitable automated biomanufacturing platform requires significant process flexibility to accommodate unique constraints of individual cell populations, in many respects similar to the flexibility required historically in MAb manufacturing strategies to accommodate variability in product titres and glycosylation profiles (6). For some cell lines that are particularly unamenable to automation, however, there has to be a tipping point at which costly process development is pushed aside in favor of focusing on first-to-market advantages that such highly unique therapies offer.
Bioprocessing Automation Benefits
Benefits of automation = improved quality + increased productivity + ease of regulatory compliance
Adding automation into long-term scale-up plans introduces another significant factor into scientific, technological, financial, and regulatory considerations. The selected automation approach should be viewed as a long-term component of a scale-up strategy that will deliver financial benefits over relatively long time periods. However, any equipment personalisation to accommodate unique product characteristics is likely to impact potential resale values. Further, well-trained operators and efficient scheduling will be required to minimize downtime due to maintenance.
Despite these hurdles, automated bioprocessing holds considerable promise for reproducible manufacturing ensuring comparability across multiple processing sites. This is achieved principally by removing manual tasks that potentially introduce high cellular variability in part due to the exposure of cells to poorly controlled bioprocess forces (5). Short-term investors may advocate more affordable manual processes and accompanying flexible staffing options; but long-term investors — including ultimate acquisition partners such as large pharmaceutical firms — will almost uniformly favor automation and scalable processes.
The adoption of cell therapy biomanufacturing automation will be limited unless product developers are able to convey their benefits and acknowledge any shortcomings. Such communication must be provided in an accessible manner to the research and small and medium-sized enterprise (SME) communities.
Value of Automation in Other Industries
From personal electronics to high-end jewelry, automation is integral for producing consistently high-quality products that address a market need, at a price the market can afford, and in sufficient volume to satisfy market demand (Table 1). Subsequently, two key metrics can be used to evaluate the success of any such automation strategy (“Evaluating Automation Success” box). One is the extent of any such process automation (on a spectrum from entirely manual to fully automated). The other is the extent to which an automation strategy enables a market (to the benefit of the entire subsequent industry) and captures a market (to the benefit of man
ufacturers and users of such technologies).
Table 1: Value of automation in other industries

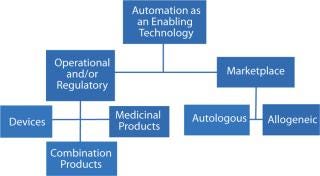
Automation as an Enabling Strategy
From a strategic viewpoint, automation is an enabling strategy. It enables the creation of markets and the commercialization of products and services in those markets. However, equally self-evident is that complete automation is not universally appropriate in all scenarios. Financial cost and process complexity are frequently cited as factors that discourage automation, particularly at small scales (7). In truth, however, these are frequently superficial observations. To understand why, consider the process of enablement itself.
The dictionary definition of enablement has two aspects — which for the purposes of bioprocessing we can use as a proxy for a factor with two dimensions (Figure 1). Firstly, it means an action or series of actions that makes something possible; for example, making a device or system operational — often within a specified system. Secondly, enablement means granting someone or something the authority or means to undertake an action or a series of actions.
Regulatory Compliance Benefits
In satisfying the regulatory aim of safe and efficacious therapies, automation aids in the control of sterility and particulates, error reduction, increased productivity and enhanced comparability (8, 9) (“Automation Benefits” box).
Sterility and Particulates: Cell therapy products cannot undergo late-stage purification or sterilization. Consequently, some manufacturers are understandably concerned about potential particulates that can be introduced during open-culture manipulations (10). For example, particulates could potentially affect the efficacy of a cellular product and/or influence the choice of administration route(s) (11, 12).
Manual handling of high-touch processing steps inevitably introduces fibers, plastic debris, and other exogenous materials into the process from gowning materials, laboratory plasticware, liquid storage bags, imprecise movements, and cleaning regimes (12). Of even greater concern is the possibility of particulate introductions by technicians themselves or surrounding environments, which could potentially compromise products — and consequently, patients’ safety.
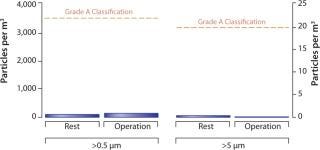
Robotic handling under aseptic conditions greatly reduces the level of possible particulates and contaminants introduced through manual culturing (13). As Figure 2 shows, the level of particulates in functionally closed robotic processing chambers is approximately 100-fold lower than requirements for a Class A enclosure. Therefore, in addition to reducing particulate burden, robotic handling reduces the likelihood of failed batches due to contamination events from standard operating procedure (SOP) breaches (14).
Error(s): Although a reduction in the likelihood of human errors is a self-evident advantage of process automation, it is especially critical in the manufacture of cellular therapies. The production process includes a large number of process steps and carries potential serious clinical risks arising from cross-batch contamination during autologous product manufacturing (13). In addition, regulatory restrictions surrounding the need for patient confidentiality make manual bioprocessing, in particular record keeping and retention, challenging (15).
Manual record keeping is poorly amenable to incumbent regulations relating electronic drug authentication at all points in the chain of custody to patients (16, 17). Manual record keeping, given labor constraints alone, is also likely to generate fewer data points for analysis and dynamic process optimization studies than automated online technologies (18). Therefore, this capability can be particularly advantageous when seeking to automate and scale-up manual laboratory-scale processes (19).
Productivity: The consistency of robotic handling supports the development and maintenance of optimal cell-processing conditions over long periods. Further, the smaller footprint of automated systems compared with manual systems increases productivity per unit facility floor area.
In terms of labor requirements, the linear relationship between labor availability and maximum theoretical facility productivity for manual processes is invalid in the context of automation. A single employee and/or team of employees can operate a number of automated systems simultaneously. Therefore, the automation of repetitive tasks increases the number of tasks completed per unit time and per unit full time employee (FTE) resource (20), supporting rapid process scalability without the need to train new staff in manual processes. Automation also supports a decrease in requirements for staff training to maintain quality management systems and good manufacturing practice (GMP) compliance as a large number of associated manual tasks are permanently automated (21).
Comparability: Achieving and demonstrating comparability in critical quality attributes (CQAs: identity, potency, purity and safety) throughout all stages of cell therapy biomanufacturing is understandably a key regulatory requirement (22, 23). It is useful to understand key drivers for the regulatory needs for comparability and ways in which biomanufacturing automation may sup
port regulatory compliance (“Hierarchy of Comparability” box).
Even the best intentions to strictly follow SOPs cannot remove the inherent variability amongst different technicians performing the “same” actions of a multistep process. Typically during manual bioprocess passaging of cells, environmental parameters such as temperature, duration of exposure to proteolytic enzymes, hydrodynamic forces, and fluid shear/stress expanded during cell resuspension are all subject to interbatch and interoperator variability (24). Such variability can detrimentally affect the consistency of CQAs and therein final products. Automation can remove the “art” of highly skilled staff with precise robotic movements and accurate timings. Such changes can improve intrabatch and interbatch comparability within and across globally located sites.
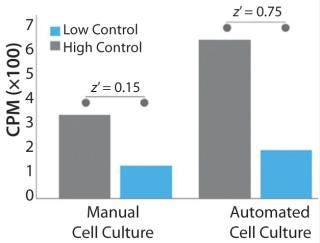
Bioprocess automation can also enable access to a greater number of techniques and tools to generate the data necessary for proving comparability in regulatory submissions. For example, due to the inconsistent cellular quality of manually cultured cells for a drug discovery assay, it was not possible to perform the radioligand binding assay depicted in Figure 3. However, this assay was successfully performed using automated techniques — further improving the dynamic range of the assay and making differentiation of the controls possible. Higher cellular reproducibility could increase the effectiveness and scope of similar types of product-release tests, thereby providing additional comparability data for regulators and improving confidence in product CQAs.
Hierachy of Comparability
Comparability to optimal in vivo conditions
Intrabatch comparability over multiple processing steps
Interbatch comparability over long periods and between multiple processing sites
Comparability in final product CQAs despite patient source material heterogeneity
Comparability throughout chain of custody inc. distribution – cell viability
Comparability in patient dosing and administration
Potential Payback Timelines for Autologous Products
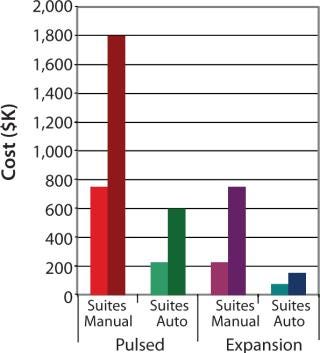
To demonstrate the cost benefit of transitioning from manual to automated culture, we investigated two models of common autologous cell therapy processes — pulsed and expansion. Pulsed models aim to simulate processes used in dendritic cell therapies in which patient immune cells are exposed to tumor lysate, peptides and/or antibodies, priming them for attack upon reintroduction (25,26,27). Expansion processes are similar to those generally used for mesenchymal scale-up. We modeled process flows within standard industry suite space and assessed the necessary full-time employee (FTE) requirements for two annual production scales at 350 and 1,000 patient doses. These two process scales can then be extrapolated for escalating commercial market penetration and/or expansion to multiple sites.
Respectively, Figures 4 and 5 show suite space and FTE costs incurred in each production model. As seen in both models, increases in the number of patients has a direct FTE impact in manual processing and a more gradual impact in automated processing, which has a lower labour requirement. The pulsed process included an additional manual interaction due to an unavoidable centrifugation step, which slightly limited the automation benefit. The expansion process alleviates staff from repetitive tasks although the lengthy culture time requires additional automation systems, therein making the net benefit relatively similar.
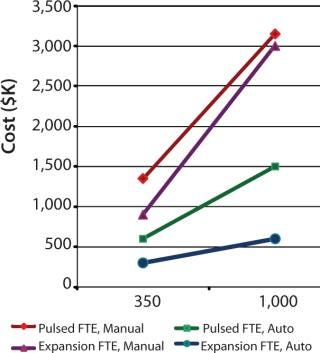
Additional manual process hindrances include the need to ensure the separation of patient samples and the resultant large “footprint” of suite space required. Separate suites and lengthy cleaning regimes between each patient are typical in manual culture. Subsequently, the number of patients that could be processed during normal working hours was considerably lower. Automated solutions avert this setback — either through the use of plastic tube welding or functionally closed processing. Further automation advantages include improved consistency of products within a site as well as amongst decentralized sites and full audit trail of product handling, both of which would aid in expediting validation.
As Table 2 shows, although automation requires significant upfront capital expenditure (an oft bemoaned complaint of SMEs), it is clear from the estimated payback periods for each process that the savings over manual quickly outweigh the risks, especially as patient numbers increase. It is important to be mindful that these savings do not account for the additional direct automation benefits of lowered product loss due to quality or contamination issues nor the reduced FTE cost effects that arise from staff illness, transition, or retraining.
Table 2: Calculation of payback timelines from savings conferred by automation versus manual processing
The Best Path Forward
The “process is the product” is a mantra that has informed the cell therapy bioprocessing community perhaps more than any other. But, to date, it has also been applied as a misnomer.
In the cell therapy industry, the strong interrelationship between regulatory strategy and financial success is undeniable. Rather less appreciated, yet of particular importance, is that “the [biomanufacturing and regulatory processes] are the product.”
The regulatory environment is driven by developments in fundamental science, just as automated processing technologies respond to needs in the translation pathway from scientific innovation to affordable and efficacious patient benefits. This triumvirate is tangible, with significant implications for industry development – positive (if managed) and negative (if continued to be neglected) (Figure 6).
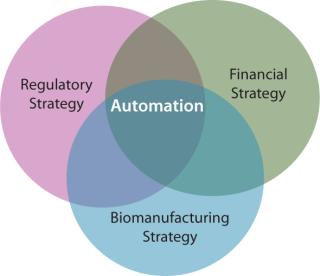
Basic scientists have valid concerns surrounding the applicability of automation in all cases, in part due to its perceived dissimilarity to optimized manual processes. Regulators are charged with the remit of protecting public health, while at the same time not hindering innovation. Investors seek the most cost-effective strategy to ensure regulatory compliance and maximize financial returns. Subsequently, the developers of automated biomanufacturing technologies face the highly formidable task of demonstrating value to all parties in the translation pathway from academic scientists to potential acquirers of a technology.
The nub of the matter is clear: Even if we are able to develop robust manual biomanufacturing strategies to enable the early stage cell therapy industry of today, will these same strategies be sufficient to enable the commercial cell therapy industry of tomorrow? There are growing signs that the financial and compliance benefits of automated biomanufacturing at production scale offer the best path forward for the sector’s success.
Therefore, it’s time to acknowledge the latest dichotomy for the cell therapy industry — beyond “autologous or allogeneic,” “transient or permanent,” “fresh or cryopreserved,” “centralized or decentralized” — and now include “automated or manual” bioprocessing. In all cases, decisions will be made severally; assessing multifactorial risks and benefits on a product-by-product basis. The most important step is to appreciate that multiple paths exist to market and the establishment of a sustainable cell therapy industry.
After all, as George Keith Funston, past head of the New York Stock Exchange said, “Automation does not make optimism obsolete!”
Disclosures
The content outlined herein represents the individual opinions of the authors and may not necessarily represent the viewpoints of their employers. D.A.B. gratefully acknowledges support from the SENS Research Foundation (Mountain View, CA, USA). D.A.B. is a stockholder in Translation Ventures Ltd. (Charlbury, Oxfordshire, UK), a company that amongst other services provides cell therapy biomanufacturing, regulatory and financial advice to clients in the cell therapy sector. D.A.B. has received hospitality C/O TAP Biosystems within the past seven years with a cumulative value of less than $10,000. D.A.B. is subject to the CFA Institute’s Codes, Standards, and Guidelines, and as such, the author must stress that this piece is provided for academic interest only and must not be construed in any way as an investment recommendation. K.E.B is an employee of TAP Biosystems (Royston, Hertfordshire, UK), which supplies automated bioprocessing solutions to the cell therapy sector. I.B.W. is a member of the World Class University Programme (Grant No. R31-10069) funded by the National Research Foundation, Ministry of Education, Science and Technology, South Korea. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed apart from those disclosed.
About the Author
Author Details
Corresponding author David A. Brindley is a researcher at Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, The University of Oxford, Nuffield Orthopaedic Centre; The Centre for Accelerating Sustainable Medical Innovations, The University of Oxford and The Harvard Stem Cell Institute; and a commercialization writer with the Centre for the Commercialization of Regenerative Medicine; [email protected]. IB Wall is a lecturer in cell therapy bioprocessing at Department of Biochemical Engineering, University College London, UK and Department of Nanobiomedical Science and WCU Research Center of Nanobiomedical Science, Dankook University, Republic of Korea. KE Bure is the North American business manager of regenerative medicine at TAP Biosystems.
1.) Gottschalk, U, K Brorson, and AA. Shukla. 2012. The Need for Innovation in Biomanufacturing. Nature Biotech. 30:489-492.
2.) Carmen, J Developing Assays to Address Identity, Potency, Purity, and Safety: Cell Characterization in Cell Therapy Process Development. Regen. Med. In Press.
3.) Aviv, A, D Levy, and M. Mangel. 2003. Growth, Telomere Dynamics, and Successful and Unsuccessful Human Aging. Mech. Ageing Dev. 124:829-837.
4.) Von Zglinicki, T. 2002. Oxidative Stress Shortens Telomeres. Trends Biochem.Sci. 27:339-344.
5.) Brindley, D. 2011. Bioprocess Forces and Their Impact on Cell Behaviour: Implications for Bone Regeneration Therapy. J. Tissue Eng. epublication 23 August.
6.) Carson, KL. 2005. Flexibility: The Guiding Principle for Antibody Manufacturing. Nature Biotech. 23:1054-1058.
7.) Fitzpatrick, I. 2008. Cellular Therapy Success Through Integrated Automation. Bioprocess Int. 6:32-37.
8.) Thomas, RJ. 2008. Cell Culture Automation and Quality Engineering: A Necessary Partnership to Develop Optimized Manufacturing Processes for Cell-Based Therapies. J Lab Auto. 13:152-158.
9.) Ratcliffe, E, RJ Thomas, and DJ. Williams. 2011. Current Understanding and Challenges in Bioprocessing of Stem Cell–Based Therapies for Regenerative Medicine. Br. Med. Bull. 100:137-155.
10.) Eichler, H. 2012. Implementation of Clean Room Facilities for the Production of Cellular Therapy Products: What Are the Consequences?. Transfus. Med. Hemother. 39:1660-1696.
11.) Clarke, D. 2012.. ISCT Process and Product Development Subcommittee Presentation.
12.) Clarke, D. 2012. Managing Particulates in Cellular Therapy. Cytotherapy 14:1032-1040.
13.) Romagnoli, L. 2011. Cell-Based Medicinal Products and the Development of GMP-Compliant Processes and Manufacturing. BMC
Proc. 5:O3.
14.) Hengster, P. 2005. Islet Isolation and GMP, ISO 9001:2000: What Do We Need? A 3-Year Experience. Transplant.Proc. 37:3407-3408.
15.) Patel, K, and N. Chotai. 2011. Documentation and Records: Harmonized GMP Requirements. J. Young Pharm. 3:138-50.
16.) Barlas, S. 2011. Track-and-Trace Drug Verification: FDA Plans New National Standards. PT (Pharmacies Tread With Trepidation) 36:203-204.
17.) European Commission.
18.) Lapteva, N, and JF Vera. 2011. Optimization Manufacture of Virus- and Tumor-Specific T Cells. Stem Cells Int.:434392.
19.) Thomas, D. 2012. Stem Cell Production: Overcoming the Technical and Commercial Challenges. Innov. Pharm. Technol. www.iptonline.com/articles/public/TAPBiosystems.pdf:66-70.
20.) Thomas, RJ, PC Hourd, and DJ. Williams. 2008. Application of Process Quality Engineering Techniques to Improve the Understanding of the In Vitro Processing of Stem Cells for Therapeutic Use. J. Biotech. 136:148-155.
21.) Prathalingam, N. 2012. Production and Validation of a Good Manufacturing Practice Grade Human Fibroblast Line for Supporting Human Embryonic Stem Cell Derivation and Culture. Stem Cell Res. Ther. 3:12.
22.).
23.) Gunetti, M. 2012. Validation of Analytical Methods in GMP: The Disposable Fast Read 102(R) Device, An Alternative Practical Approach for Cell Counting. J. Translational Med. 10:112.
24.) Veraitch, FS. 2008. The Impact of Manual Processing on the Expansion and Directed Differentiation of Embryonic Stem Cells. Biotech. Bioeng. 99:1216-1229.
25.) Hsu, FJ. 1996. Vaccination of Patients with B-cell Lymphoma Using Autologous Antigen-Pulsed Dendritic Cells. Nature Med. 2:52-58.
26.) Nestle, FO. 1998. Vaccination of Melanoma Patients with Peptide- or Tumorlysate-Pulsed Dendritic Cells. Nature Med. 4:328-332.
27.) Thurner, B. 1999. Vaccination with Mage-3a1 Peptide–Pulsed Mature, Monocyte-Derived Dendritic Cells Expands Specific Cytotoxic T Cells and Induces Regression of Some Metastases in Advanced Stage IV Melanoma. JEM 190:1669-1678.
28.) Christensen, CM, and M. Raynor. 2003.The Innovator’s Solution: Creating and Sustaining Successful Growth, Harvard Business School Press, Boston.
29.) Magretta, J. 2002. Why Business Models Matter. Harvard Bus. Rev. 133 80:86-92.
30.) Spear, S, and H. Kent Bowen. 1998. Decoding the DNA of the Toyota Production System. Harvard Bus. Rev..
You May Also Like