Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
Abiological measurement of drug activity is perhaps the most critical step in the series of tests required for product release both for clinical trials and the market. This evaluation plays an important role in the stability assessment of drug candidates. According to the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Q6B document, measurements of biological activity can be performed in defined animal models that demonstrate a measurable physiological change in response to application of the drug (1). But animal studies are costly, time-consuming, and known to have high degrees of variability, thereby raising questions about their effectiveness. In addition, the reduction, refinement, or replacement of such in vivo assays is a common goal of the animal welfare community health authorities, and some pharmaceutical manufacturers (2, 3, 4).
PRODUCT FOCUS: ALL BIOLOGICS
PROCESS FOCUS: ANALYTICAL
WHO SHOULD READ: PROCESS, DEVELOPMENT, ANALYTICAL, QA/QC
KEYWORDS: PRIMARY CELLS, HUVEC, CRYOPRESERVED, BIOASSAYS, POTENCY, GMP, VALIDATION, LOT-RELEASE, STABILITY
LEVEL: INTERMEDIATE

In vitro bioassays using immortalized cell lines provide an alternative approach to animal-based bioassays. Cell lines are easy to use, grow continually (dividing indefinitely in vitro provided that correct culture conditions are maintained), and are acceptable as a standard for cell-based assay development. But immortalized cell lines (also known as transformed cells) have limitations as a result of changes in their growth properties.
Primary cells express characteristics that more closely represent in vivo behavior than do transformed cell lines and are an excellent alternative to animal experimentation. They can be used to develop in vitro potency assays that more closely reflect the mechanism of action of a therapeutic molecule. Unlike immortalized cell lines, however, primary cells have limited growth potential even under optimal growth conditions and eventually senesce and die. Thus, maintaining them for experimental purposes is a major challenge. Using primary cells for an in vitro bioassay requires frequently thawing and culturing a new vial of cells, which is impractical in a routine quality control (QC) testing laboratory. In addition, the characteristics of primary cells can change with each subsequent passage (5, 6).
To overcome such limitations, we have developed a novel approach for developing bioassays based on ready-to-use cryopreserved primary cells. We have successfully used this method for product lot–release and stability assessment in a QC environment under good manufacturing practice (GMP) conditions. We believe that our approach can be applied to any cell type and that it offers significant advantages over traditional cell-based assay development and routine use.
Here we present a case study describing a bioassay we have developed with ready-to-use cryopreserved primary cells. It highlights the multiple benefits of using ready-to-use cryopreserved cells instead of fresh cells. We also discuss challenges associated with preparing and qualifying primary cell banks, such as ensuring stability over time and conducting effective technology transfer to commercial QC testing laboratories located in several countries. The data discussed herein were presented for the first time at the “Bioassay 2010: Scientific Approaches and Regulatory Strategies” conference held in Bethesda, MD, in 2010 and at the “Informa 10th Annual Biological Assays” conference held in Berlin, Germany, in 2011.
Current Challenges with Traditional Approaches
Current approaches to cell-based assay development rely on continuous cell culture. The process requires considerable operator support with maintenance and monitoring of culture conditions and associated data recording. Growth rates can differ among different cell types postseeding. That can restrict days of the week on which an assay can be performed to those days when flasks of cells are ready for harvesting.
As the number of drug pipeline molecules increases, the number of cell-based assays to be developed for GMP lot-release/stability or characterization also rises. The number of cell lines to maintain can become unmanageable because of limited (or lack of) laboratory space, available incubators, specialized cell-culture facilities, and resources.
An even greater challenge is the development of robust methods using primary cells. Such cells can exhibit significant donor-to-donor variability, are susceptible to damage during frozen storage, and lose characteristics and responses over a short period of time in culture. All those factors make the use of primary cell–based bioassays unsuitable for a QC environment.
Ready-to-Use Methodology Principles and Benefits
Despite such challenges, we have successfully developed robust bioassays for QC to support clinical and commercial products using ready-to-use cryopreserved primary cells. Our novel approach consists of expanding cells to an optimal passage level and generating a large ready-to use cryopreserved cell bank. On the day an assay is performed, a vial of cryopreserved cells is thawed and resuspended in assay medium, and then a cell suspension is directly dispensed in assay microplate wells (Figure 1).
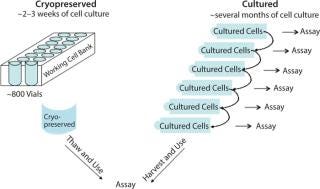
Our approach eliminates the need to maintain cells in culture continuously, to frequently reinitiate cell culture (because primary cells have very limited passage number), and to harvest a flask before initiating an assay. Advantages include
significant increase in efficiency (fewer personnel needed for cell maintenance and media preparation)
increased consistency in method performance (cells are frozen at the same passage)
added flexibility (assays can be performed any day of the week)
significant cost reduction for cell culture media, reagents, and vessels
easier method transfer (handling cells for thawing, culturing, and freezing can account for many problems at recipient laboratories).
Points to Consider in Ready-to-Use Methodology
Several factors should be considered before selecting ready-to-use cryopreserved cells for a bioassay over fresh cells from contin
uous cultures. It is first critical to identify optimal growth and freezing media, optimal cell passage number, freezing and thawing conditions, centrifugation speed for pelleting cells, and cell density in vials. Some cells are more sensitive than others to thawing duration and temperature.
If banks will be used by multiple laboratories, it is preferable to make large banks of at least 600–800 vials. Each vial should contain a cell number sufficient for one assay. One should demonstrate consistency in multiple frozen cell bank preparations and ensure that cells frozen in vials are stable over time.
It can take a considerable amount of time to establish the above conditions to successfully bank cryopreserved cells.
Case Study
Here we discuss the development, optimization, and validation of a potency assay that was developed for an anti-vascular endothelial growth factor (VEGF) therapeutic monoclonal antibody (MAb) using ready-to-use cryopreserved primary human umbilical vein endothelial cells (HUVEC). The HUVEC potency assay measures the ability of anti-VEGF MAb to inhibit VEGF-induced HUVEC proliferation. HUVEC are a well-known primary cell type used to examine the effects of angiogenic factors (7, 8). These cells are ideal for assessing inhibition of VEGF-induced proliferation assay. Before bank generation, a lot of HUVEC is first characterized for suitability for bioassay performance.
Assay Procedure: The assay is conducted in 96-well tissue culture microplates. We mixed different concentrations of anti-VEGF reference standard or samples with a fixed amount of recombinant human VEGF (rhVEGF) in a human endothelial cell serum-free medium (from Gibco). We added 50 µL of the rhVEGF/anti-VEGF mixtures to the microplates, then added 50 µL of HUVEC suspension (10,000 cells/well). The plates were incubated at 37 °C, 5% CO2 in a humidified incubator for four days. Afterward, we added 25 µL of the redox dye, alamarBlue and incubated the mixture for an additional six hours. The relative number of viable cells was measured based on the uptake and metabolism of alamarBlue (9). Results were expressed in relative fluorescence units (RFU).
We used a parallel line analysis program to estimate test-sample potency relative to the reference standard. Inhibition of VEGF-induced HUVEC proliferation is proportional to anti-VEGF concentrations. For system suitability, we added an assay control consisting of a sample of anti-VEGF different from the reference standard to each plate. Its primary function is to provide a criterion for judging the acceptability of results (10). For a sample result to be valid, the calculated activity of the assay control has to be within ±20% of targeted potency. We set acceptance criteria range based on development data.
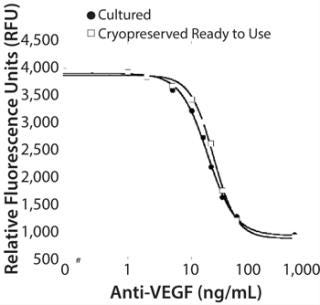
Figure 2 compares dose–response curves for ready-to-use cryopreserved cells to freshly passaged cells. Results show similar responses, which indicate that cryopreserved ready-to-use HUVEC do not affect assay performance.
Cell Bank Preparation: We purchased primary-pooled HUVEC from Cascade Biologics–Life Technologies and grew them in a medium especially formulated for HUVEC supplemented with growth factors necessary for endothelial cell proliferation. Because the life span of HUVEC in culture is limited after which they lose their endothelial properties (8, 11, 12), we tested HUVEC in the potency assay for their response to VEGF at each passage to identify passage range of optimal response. We chose a passage level for maximum cell yield while maintaining endothelial properties. Once the optimal passage level was selected, we expanded HUVEC to that passage level and cryopreserved them in aliquots at the optimized density to maintain viability postthaw. One vial of cryopreserved cells was thawed for use in each assay.
Initially, we generated five HUVEC banks in-house using five different combinations of freezing media containing basic media, dimethyl-sulfoxide (DMSO), and fetal bovine serum (FBS). We evaluated cryopreserved banks for cell viability pre-and postcryopreservation and for activity (assay control quantitation). Results in Table 1 indicate that freezing medium A (basic medium 1 supplemented with 10% FBS and 5% DMSO), B (basic medium 1 supplemented with 5% DMSO), and C (basic medium 2 supplemented with 10% FBS and 10% DMSO) maintained viability of the HUVEC comparably (83–87%) over a 24-month storage time. Results showed no impact on assay performance as indicated by comparable assay control quantitation of 99%, 104%, and 97% relative potency, respectively. By contrast, freezing medium D (basic medium 3, supplemented with 10% FBS and 10% DMSO) did not maintain cell viability (57%).
Table 1:
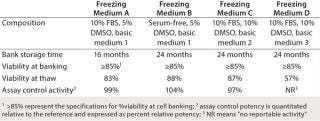
Because the difference between freezing media C and D is the composition of basic medium, these data highlight the importance of selecting the proper basic medium for long-term storage of cryopreserved HUVEC banks. The difference in media composition could not be determined because the composition of basic medium 3 was vendor proprietary and therefore unknown. Table 2 shows that cryostorage incubation time in freezing medium C of up to eight years in liquid nitrogen did not influence cell viability (90%) or assay performance (control quantitation of 101% relative potency).
Table 2:

Extensive prework was required to establish optimal conditions for banking HUVEC (data not shown nor discussed here). We found the critical elements to be selection of optimal passage level; growth medium; freezing medium including the concentration of
cryoprotectant (DMSO); and optimal freezing protocol including number of cells per vial and step-down cryofreezing.
HUVEC Bank Evaluation and Qualification: Because HUVEC are a critical reagent for the potency assay, it was important to show reproducibility of bank preparation by different facilities and to demonstrate multisource availability. We purchased vials of primary HUVEC from an external vendor and expanded them to generate three ready-to-use cryopreserved banks. Bank lots 1 and 2 were prepared in-house. Bank lot 3 was prepared by an external vendor.
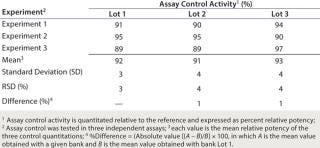
We set the following acceptance criteria to qualify the three HUVEC banks: Amplitude of signal in response to VEGF must be at least threefold, and assay control quantitation must fall within the established acceptance range of ±20% comparing a new bank and a bank currently in use. As Table 3 shows, assay control quantitation ranged from 91% to 93% relative potency with relative standard deviation (RSD) <4%. The percent difference was 1% when the value obtained with bank lot 1 was compared with the values obtained with bank lots 2 and 3.
Table 3:
Based on these results, banks of ready-to-use HUVEC prepared in different facilities from primary cells purchased from the same vendor can be used with no influence on assay performance. In addition, we demonstrated that banks prepared in-house from HUVEC purchased from three different vendors also met acceptance criteria (data not shown). These results were reassuring because it is important to have more than one supplier of cells available.
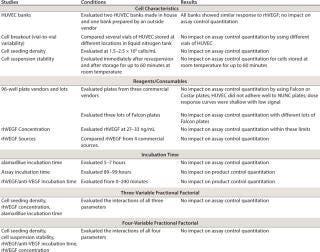
Robustness Studies: Before deciding to select and validate this bioassay as a potency assay and transfer it to other commercial testing laboratories, we evaluated its robustness around the following key parameters: cell breakout (vial-to-vial variability), cell seeding density in the assay plate, cell suspension stability, plate vendors, rhVEGF concentration, rhVEGF source, alamarBlue incubation time, assay incubation time, and reagent stability. We varied each parameter and tested it independently.
We also conducted two fractional factorial experiments in which several parameters varied from target conditions were tested together in a single assay to evaluate possible effects of interaction of those parameters on robustness. We considered a method parameter to be robust if the assay control quantitation was within ±20% of its established mean. Table 4 summarizes results of the robustness studies. The assay had suitable robustness within the parameters and ranges tested.
Table 4:
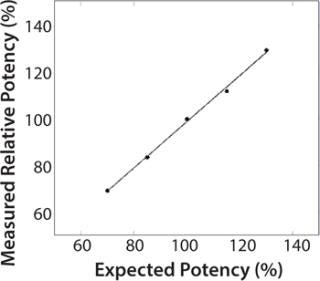
Validation Studies: We validated the potency method using ready-to-use cryopreserved primary HUVEC for commercial use in accordance with ICH Q2(R1) (13) over a potency range of 70–130% of target. Tables 5 and 6 show results of those studies. The method was accurate with recovery ranging between 95% and 108% and precise with interassay RSD of 4–6% (Table 5). We determined linearity of potency measurement using five concentrations corresponding to 70, 85, 100, 115, and 130%. Figure 3 shows measured relative potencies versus expected potencies and the applied linear regression. Results showed a correlation coefficient r = 0.999, slope = 1.04, and y-intercept = –6.28. Recovery at each concentration ranged from 94–97% (data not shown). The r value in combination with the sample recovery data demonstrated that the method had an acceptable degree of linearity. Table 6 shows that the method was stability-indicating because it could detect changes in activity of samples subjected to heat, light and basic pH.
Table 5:

Table 6:

Next Challenge: Method Transfer to Other Testing Laboratories
We transferred our validated HUVEC antiproliferation method to multiple commercial testing laboratories. Because the originating development laboratory could not generate cell banks for all commercial testing laboratories, we identified a commercial vendor that, from that point on, could generate all banks for all sites. The vendor’s responsibilities were to prescreen source cells for receptor level and responses to ligand (VEGF); to provide a certificate of analysis for mycop
lasma, virus, and viability tests; and to distribute cells to testing laboratories after completion of cell bank qualification performed by the originating laboratory. Over time, we identified additional vendors that could generate large banks.
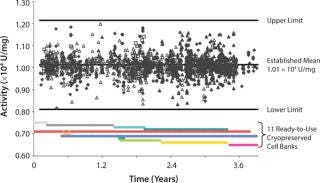
Our HUVEC-based method has been performed as a lot-release and stability test at a large number of testing laboratories at different locations for 10 years. The method has been monitored from the beginning across sites using assay control trending charts. The chart in Figure 4 trends the assay control quantitation from six testing laboratories over time and covers the use of 11 ready-to use HUVEC banks. Results show no obvious trend in the data observed over four years. Most results were within ±20% of the established mean of 1.01 × 104 units/mg. The mean activity from 1,732 values generated by 28 analysts and collected over four years was similar to the expected and established mean of 1.01 x 04 U/mg, with 4% RSD. That is consistent with the precision observed in the recovery study (4–6% RSD), with an assay success rate of 93% of assay plates meeting system suitability. These performance parameters are indicative of a very consistent method performance.
Future Work
Ready-to-use cryopreserved cell technology is a practical and novel approach for cell-based assays used for drug development. This approach was originally developed for anti-VEGF MAb a decade ago and is now an established technology that we use for several validated potency assays. Ready-to-use vials of cells are thawed and plated directly into an assay plate, greatly simplifying assay performance and reducing the number of steps, overall operator time, and costs.
Although our approach improves efficiency, consistency, flexibility, and economics, it also presents specific challenges and points to consider to end-users as presented here. That is especially true if a cell-based assay is to serve as a potency assay in support of GMP product release/stability testing in a QC laboratory and will be transferred to multiple sites.
Proper cell bank documentation is critical for each bank preparation process as recently published (14). We strongly recommend detailed document for each ready-to-use cell bank preparation, including source of cells, method used for propagation, preparation and qualification, number of cells and volume in each vial, certificate of analysis, and storage conditions.
Over the years, we have successfully extended the applicability of ready-to-use cryopreserved cell methodology to several mammalian cell types, both suspension and adherent. To meet increasing demands for cell-based assay development with numerous different cell types (both primary and immortalized), cryopreserved ready-to use cells are currently used in more than 30 bioassays. Culturing large number of cell types over extended periods of time is challenging and costly. It also requires additional personnel and cell culture facilities, increases the risk of contamination, and is simply becoming unmanageable. Our ready-to-use approach provides a solution for those challenges.
As expected, our approach has significantly improved method transfer. It is well known that some major difficulties in transferring methods using passaged cells relate to cell performance in a recipient laboratory, which can lead to significant delays in assay transfer. The ready-to-use cryopreserved cell approach eliminates the need for cell culturing and allows for more consistent cellular response because cells are frozen at the same passage. It has greatly expedited bioassay transfers due to consistent assay performance.
We are currently evaluating several aspects of cell bank preparation, including use of larger cell culture vessels to scale up the number of banks and vials per bank that can be produced for distribution to a growing network of testing sites; incorporation of automation in cell dispensing; and development of new freezing methods and storage conditions.
About the Author
Author Details
Corresponding author Hélène Gazzano-Santoro, PhD, is a director in the Analytical Development and Quality Control department at Genentech, a member of the Roche Group, 1 DNA Way, South San Francisco, CA 94080; [email protected]. Linda G. Chan is a senior QC associate in the Biological Technologies group within the Analytical Development and Quality Control department. Mimmi S. Ballard is a graduate student In the Department of Molecular, Cell, and Developmental Biology at the University of California, Santa Cruz. Judy C. Young, PhD, is a scientist in the Biochemical and Cellular Pharmacology department at Genentech, a member of the Roche Group.
1.) ICH Q6B 1999. Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products.
2.) Castle, P 2007. Replacement, Reduction, Refinement (3Rs) Animal Welfare Progress in European Pharmacopoeia Monographs. Pharmeuropa 19:430-441.
3.) 2008.Alternatives to Animal Testing: New Approaches in the Development and Control of BiologicalsProceedings of the International Symposium, EDQM/Council of Europe, Dubrovnik.
4.) 2009.Information Requirements of the Animal Welfare Act Code of Federal Regulations, Animal Welfare.
5.) Bala, K, K Ambwani, and NK Gohil. 2011. Effect of Different Mitogens and Serum Concentration on HUVEC Morphology and Characteristics: Implication on Use of Higher Passage Cells. Tissue and Cell 43:216-222.
6.) Unterluggauer, H. 2007. Identification of Cultivation-Independent Markers of Human Endothelial Cell Senescence In Vitro. Biogerontol. 8:383-397.
7.) Montesano, R, M Pepper, and L Orci. 1990. Angiogenesis In Vitro: Morphogenetic and Invasive Properties of Endothelial Cells. News Physiol. Sci. 5:75-79.
8.) Ferrara, N. 1991. Purification and Cloning of Vascular Endothelial Growth Factor Secreted by Pituitary Folliculostellate Cells. Methods Enzymol. 198:391-405.
9.) Page, B, M Page, and C Noel. 1993. A New Fluorometric Assay for Cytotoxicity Measurements In Vitro. Int. J. Oncol. 3:473-476.
10.) Gazzano-Santoro, H, C Broughton, and D Schmalzing Agalloco, J and FValidation of Analytical Procedures and Physical MethodsValidation of Pharmaceutical Processesthird edition, Informa Healthcare:655-663.
11.) Maciag, T Jaffe, E. 1984.Biology of Endothelial Cells, Martinus Nijhoff Publishers, Boston:87.
12.) Maier, J. 1990. Extension of the Life Span of Human Endothelial Cells by an Interleukin-1α Antisense Oligomer. Science 249:1570-1574.
13.) ICH Q2(R1) 2005. Validation of Analytical Procedures — Text and Methodology.
14.) Menendez, A. 2012. Recommendations for Cell Banks Used in GXP Assays: Preparation, Characterization, and Storage. BioProcess Int. 10:26-40.
You May Also Like