Outsourcing to Enhance Assurance of Supply: Application of Counterintuitive Supply Chain Strategies — A Case Study
Single-use technologies have transformed biopharmaceutical manufacturing by providing tremendous and proven opportunities to reduce costs, improve flexibility, and shorten cycle times. The expansion of such technologies into commercial production has naturally raised new challenges for both end users and suppliers, thus driving the need for a critical look at risks associated with their use. End users now face a new challenge: how to assess their own supply chains for robust assurance of supply. What is the suppliers’ responsibility in addressing such concerns? End users must rely on their single-use suppliers as the provider of their process train components. How should users ensure process safety and efficiency when such aspects are transferred to a single-use supplier?
The risk of relying on suppliers has long been a topic of discussion when end users considered the “single-use decision.” Many risk assessments so far have led to incomplete strategies. The first step is a different and more comprehensive approach to defining assurance of supply. A more robust definition includes business continuity, consistent quality, and robust change control. Quality agreements and dual sourcing traditionally have been attempted to mitigate associated risks, but such solutions have shown weaknesses and been unable to eliminate supply chain risk.
Traditional single-use business models also have made those efforts difficult, with extraordinarily complex supply chains often involving unqualified/uninterested suppliers. End-users’ traditional approaches can handle only one or two aspects of single-use supply topic. That illustrated a crucial need for a novel, end-to-end comprehensive approach to that addresses all aspects of ensuring supply, beginning with suppliers buried deep within the chain. Sartorius Stedim Biotech has developed a comprehensive framework to ensure supply of films, components, filters, and bags with consistent quality, robust change management, and business continuity.
Quality by design (QbD), material science, film extrusion know-how, and bag-making expertise are the foundations of this new level of supply assurance, bringing complete control and transparency over the entire supply chain for Flexsafe, Flexboy, and Celsius bag families. Combining vertical integration of critical process steps with a smart outsourcing strategy involving industry-leading partners provides end users with the level of confidence they need for implementing single-use technologies in their own manufacturing processes.
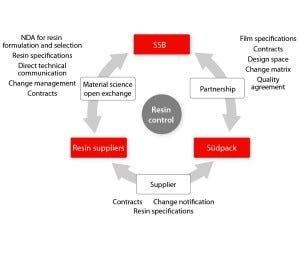
Figure 1: Three-way relationship required to establish a new paradigm of assurance of supply of film for single-use systems
New Supply Chain Strategy
Assurance of supply needs to be built on close partnerships, supply contracts, quality agreements, and transparency to provide control over an entire manufacturing process — in our company’s case, from resins to final single-use products. Resin specifications, process qualifications, and process controls established at all stages of the manufacturing process ensure quality, change control, and business continuity.
In close collaboration with resin and film suppliers, polymer scientists and biologists have followed a scientific material science and QbD program to develop a completely new polyethylene film (S80) for consistent performance with Flexsafe bags for all bioprocess unit operations and applications. The same approach has been implemented for S71 film, which is used in the Flexboy and Celsius bag families. Reliability of the supply chain for our company’s single-use products is based on partnerships and agreements with its suppliers as well as complete understanding and control of the manufacturing process from resin and film extrusion to final, sterile bioprocessing bags. Specifications and controls established for the resins provide change control and consistent critical quality attributes (CQAs) and performance of films. Quality agreements and safety stocks guarantee consistent quality and business continuity for critical raw materials and components of our company’s single-use predesigned solutions.
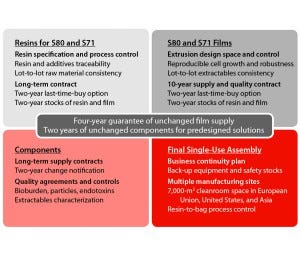
Figure 2: Key elements of supply assurance for the major stages of single-use systems manufacturing
Assurance of supply relies on a long-term contract with our film supplier. A large extrusion capacity allows that company to adsorb the double-digit growth demands for single-use bags.
Partnership, Material Science, and QbD: Successful implementation of single-use technologies by end users is possible when their relationships with suppliers of those technologies are brought to another level based on open science and risk management. Supplier reliability is demonstrated by an ability to establish robust and trustworthy relationships at all stages of a complex supply chain. In partnership with film manufacturer Südpack, Sartorius Stedim Biotech established direct contacts with selected industry-leading suppliers of plastic resins. Direct access to raw-material suppliers provides an unprecedented level of information on the initial resin and additive package formulation, which is a key part of this new strategy to achieve complete control of the entire single-use bag supply chain, from raw materials to finished products. The partnership with Südpack allowed our company to access resin suppliers and combine material science and QbD expertise.
Long-Term Supply Contracts, Safety Stocks, and Quality Agreements: For end users, single-use implementation should come from a formal partnership with a supplier that can ensure complete control of its own complex supply chain. Sartorius Stedim Biotech has built open, trustworthy, and science-based partnerships and relationships with critical suppliers. Robustness of change control and business continuity planning for raw materials and components up to final single-use products are guaranteed by long-term supply contracts and quality agreements with all critical suppliers and partners. A large manufacturing network allows for fast transfer and capacity balance across multiple sites and provides a robust business continuity plan in case of disaster.
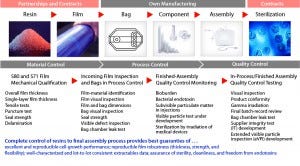
Assurance of Supply, AoS, Qualification and Control of Single-use Manufacturing Process
That approach is the best risk-based plan to cover commercial risks of single sourcing. With an enhanced assurance of supply level that includes business continuity planning, end users can be assured of continued single-use supplies even in the unlikely event of fire, flood, and so on. Not even dual sourcing can achieve the same level of quality consistency.
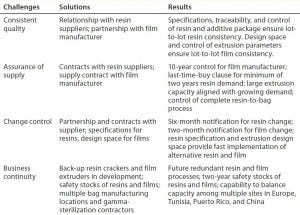
Table 1: Global approach to supply assurance: business continuity, consistent quality, and change
Enhanced Quality Assurance and Assurance of Supply
End users are ultimately responsible for their own process validation and drug-substance/drug-product manufacturing. They need to take into account the complexity of their own raw materials (e.g., media, consumables, and equipment) in process qualification and daily production steps. For single-use technologies, the entire supply chain is complex. It first requires in-depth material science and film expertise to achieve consistent quality attributes for plastic film, bags, and final, sterile single-use assemblies.
One key aspect of single-use quality assurance is collaboration with the manufacturers of resins and additive packages used for extrusion of the base film. Working directly with the suppliers of raw materials and plastic resins is of paramount importance to establish specifications, operating ranges, and process controls. This provides full understanding and traceability of the initial resin and additive package used in making a film.
Second, ensuring robust product quality requires full understanding of the complete manufacturing process. That includes definition of operating ranges and implementation of multiple controls to guaranty lot-to-lot performance consistency for the film, bag, and final assembly. This overall knowledge and understanding of the entire process from resin to final product can be achieved only with strong relationships and partnerships between the single-use bag manufacturer, raw material suppliers, and the film manufacturer.
With that knowledge of resin, additive, and catalyst formulation as well as the critical process parameters (CPPs) for film extrusion, it is possible to understand what CPPs influence the CQAs of the final product. That QbD approach helps develop more detailed resin specifications for product-contact layers and film backbones to provide consistent lot-to-lot film performance and bag quality attributes such as cell-growth performance, robustness, and extractables profiles.
Detailed formulations of the different compounds that enter into the manufacturing of our company’s films provide consistent and relevant extractable profiling, data reporting, and leachables validation support. Knowing what films are made of helps us interpret extractables data precisely and accurately while ensuring the repeatability (and hence long-term relevance) of those data. This overall approach has been implemented to consolidate the supply of existing S71 ethylene vinyl acetate (EVA) film used for Flexboy and Celsius bags as well as development of the new S80 polyethylene (PE) film used for the new Flexsafe bags.
Qualification and Control of Manufacturing
Based on the QbD approach, qualification of single-use bioprocessing is not restrained to the single-use product itself, but rather extends to the whole supply chain — from resin manufacturing and film extrusion to final single-use assemblies.
Robust Change Control: Our company’s long-term contracts with resin and film suppliers involve a six-month notification of changes in raw materials and/or manufacturing processes for resin and two-year notification for film. In addition, a last-buy option of unchanged material for two years of resin demand allows a comfortable time period of up to four years for assessing and studying the effects of changes on end users.
It is important to note that establishment of resin specifications provides more robust resin change control and facilitates change management than general trademarks can. With the established specifications and an understanding of resin CQAs, validation of an equivalent resin becomes a reality. Releasing and controlling resins with specifications, not trade names, enhances the quality of films, facilitates change management, and improves long-term assurance of supply. That allows for fast implementation of alternative resin suppliers in the unlikely event of change or discontinuation of some raw materials.
In addition, the established design space for a film extrusion process provides for fast and easy-to-validate implementation of new extrusion equipment if or when a change or capacity ramp-up is required.
Business Continuity Planning
Business continuity plans and assurance of supply often get considered together or even as interchangeable. Supply assurance means providing products consistently under their original specifications and quality requirements assuming normal operations. That comes from long-term contracts, large manufacturing capacity for all process steps, and control of the entire manufacturing process from raw materials and films to final, sterile products.
Additionally, business continuity planning covers unexpected and unlikely events such as discontinuation of raw materials or operational shut-downs because of earthquake, fire, flood, storms, or other potential disasters. This is best achieved by ensuring that every critical process step can be run using at least two different pieces of equipment in two different manufacturing locations. When redundant process equipment is unavailable, another alternative is to maintain safety stocks that will cover demand for the period that it would take to build and qualify new equipment or production lines in new locations.
Multiple bag-making equipment and assembly lines installed in multiple manufacturing locations ensure business continuity for final bag assemblies, as well. Also, multiple gamma-sterilization sources and suppliers worldwide is essential to providing final assurance of sterility.
A Strategy for Success
End users of single-use technologies are facing new challenges. Selection of suppliers is of utmost importance because the responsibility and safety of their process is partly transferred into the hands of those suppliers. Thus, end users need a robust and risk-based approach to selecting the right disposable supplies and suppliers. In recent years, our company has worked on a strategy to enhance quality, assurance of supply, and change control for its single-use bags and intelligent solutions made of bags, filters, tubes, connectors, sensors, process analytical tools, and hardware. The strategy described herein is based on
partnerships and long-term contracts with suppliers, which provides control of both raw materials and films and the extension of resin and film extrusion capacity
continuous expansion of our own manufacturing capacity in multiple sites for a flexible business contingency plan.
That strategy provides for complete control and an unprecedented level of supply assurance to end users for safe and robust biopharmaceutical manufacturing.
Further Reading
Ishii-Watabe A, et al. Approaches to Quality Risk Management When Using Single-Use Systems in the Manufacture of Biologics. AAPS PharmSciTech 16(5) 2015: 993–1001.
Cappia J-M. Enhanced Assurance of Supply for Single-Use Bags: New Flexsafe Bag Family. BioProcess Int. 12(7) 2014.
Barbaroux M, Gerighausen S, Hackel H. An Approach to Quality and Security of Supply for Single-Use Bioreactors. Disposable Bioreactors II: Volume 138 of Advances in Biochemical Engineering/Biotechnology. Eible D, Eible R, Eds. Springer: Berlin–Heidelberg, Germany, 2014: 239–272.
Sette A, Barbaroux M. Properties of Materials Used in Single-Use Flexible Containers: Requirements and Analysis. BioPharm Int. November 2006.
Corresponding author Elisabeth Vachette is senior global product manager for Flexel and Flexsafe at Sartorius Stedim FMT SAS, Z.I. des Paluds, Avenue Jouques, CS 91051, 13781 Aubagne Cedex, France; 33-442-84-5600, fax 33-442-84-5619; [email protected]; www.sartorius-stedim.com. Paul Priebe is fluid management technologies marketing director for North America ([email protected]); Carole Langlois is senior global product manager for Flexboy and ATS ([email protected]). Luke Heaven is fluid management technologies marketing director for Europe, Asia, NEMEA, and Cuba ([email protected]). And Jean-Marc Cappia is vice-president of marketing for fluid management technologies ([email protected]). Flexel, Flexsafe, Flexboy, and Celsius are all registered trademarks of Sartorius Stedim Biotech.
You May Also Like





