- Sponsored Content
- Cell Therapies
Improving Titer, Quality and Efficiency of AAV Manufacturing and Production by Optimizing Osmolality
October 14, 2022
Sponsored Content
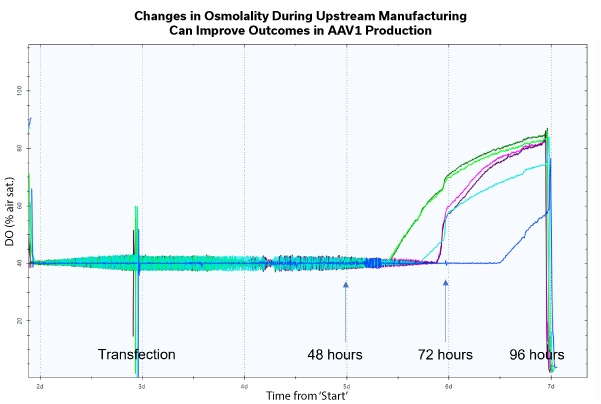
The rate of innovation within Cell and Gene Therapy is incredible and will continue to grow at an exponential rate as new methodologies are developed. With the novel techniques, come new and improved manufacturing procedures for sought after therapies. The use of viral vectors is now a common approach within the sector, but there are still difficulties when scaling up for bioprocessing. An example being a recovery range of 5-30%, a less than optimal result within the manufacturing process.
When speaking to process development scientists, it is clear the 3 main pain points within their manufacturing and production processes are: low yield, the quality of that yield, and the efficiency of their overall workflow. Increasing the manufacturing capacity, improving the production yields and the improvement of the supply chain logistics, can make these therapies accessible to larger patient populations, all with hopefully a sustainable cost. So, what is the key to improvement in all areas? The answer being the implementation of a high-quality process design and the inclusion of process parameters to ensure robustness and reproducibility at all points within the workflow. Osmolality, a measure of solute concentration, can have many applications throughout the entirety of the cell and gene therapy process. There is now clear evidence to show how the manipulation of this critical process parameter can improve overall outcomes. The study presented here, undertaken by Cell and Gene Therapy Catapult, provides preliminary evidence for impact on improving titer, quality and efficiency of AAV manufacturing and production by optimizing osmolality due to a timed osmolality shift with and without a feeding step.
About the Author(s)
You May Also Like





