- Sponsored Content
Exploring the Science Behind Single-Use Container–Closure Integrity Assurance
Sponsored Content
Failures in the integrity of single-use systems during commercial manufacturing can cause a number of serious problems for biomanufacturers. A loss of system integrity during processing can allow environmental contaminants that can be dangerous for patients (e.g., microbes) to enter a process. Biopharmaceuticals and their intermediates can be highly potent or even infectious agents, so an integrity failure can jeopardize the safety of operators. In severe cases when biomanufacturers cannot ensure the quality of drug products for fear of a contamination, the supply of life-saving medicines to patients can be restricted. Finally, significant costs can be associated with product losses and quality investigations that arise because of leaks within systems.
It is perhaps unsurprising that single-use container–closures are coming under increasing scrutiny from regulators. At many industry meetings, participants from suppliers, end users, and regulators discuss the topic.
For example, the US Food and Drug Administration (FDA) and the American Society for Testing and Materials (ASTM) ran a workshop in 2016 exploring issues relating to using single-use systems in fill–finish applications. The outcomes of that workshop reflected much discussion happening more broadly such as the recommendation that development of physical integrity tests should be correlated to microbial ingress. Suppliers and biomanufacturers must share the responsibility for container–closure integrity assurance. Reflecting that shared responsibility, workshop participants concluded that packaging integrity validation is needed at both the supplier site and (following shipment and installation) at the biomanufacturing location. It was also proposed that defect sizes that could allow bacterial ingress under process conditions into the critical flow path downstream of sterilizing-grade filters should be identified by microbial challenge testing — thereby allowing a physical integrity test to be developed.
Despite the increasing regulatory scrutiny, the industry lacks a general understanding about the size of defects in single-use systems that can allow for liquid leaks or microbial ingress under processing conditions. Biomanufacturers often are confused about commercially available integrity-testing technologies for single-use systems and the meaning of the results that they generate. Here we describe our current understanding of these subjects with a view to advancing discussion within the industry regarding how best to ensure the integrity of single-use systems.
Understanding Liquid Leaks and Microbial Ingress Mechanisms
Liquid leakage and microbial ingress both depend on process conditions, fluid attributes, and the size of container defects. The maximum allowable leakage limit (MALL) is defined by USP <1207> for maintaining the microbiological integrity of sterile packaging as the largest defect that is tolerable and poses no risk to product safety. Determining the MALL for single-use systems is fundamental to development of physical test methods with detection limits that are correlated both to liquid leaks and microbial ingress.
We have reviewed literature in this field, concluding that there is strong relationship between the size of defects that allow leaks and those that allow microbial ingress. Some scientists have found that ingress could not occur without liquid flow through a defect. Whether fluid flows through a defect depends on pressure and surface tension. Water in a representative bioprocess bag (e.g., a hanging Flexboy® 20L or Flexsafe® 500L installed in a Palletank) has a liquid height of ~630 mm. The pressure of water under static conditions does not exceed 65 mbar at that liquid height. Using mathematical models from the literature, we can predict that leaks will occur under liquid-storage conditions with defects of 15 μm or larger.
That situation becomes more complex when we consider critical applications. Our analysis shows that pressure pulses of ±0.3 bar can occur during transportation of bags by truck or by air. However, because the headspace in liquid-filled bags usually is insignificant, and the liquids they contain are noncompressible, the differential pressure during transportation does not affect pressure in the bags to significant extent. It is our belief that pressure pulses during shipping do not significantly affect the MALL. What should be of greater concern to bioprocess engineers transporting liquids in single-use systems, however, are accelerations and shocks during shipment, which can be up to 20g. The MALL for bags exposed to such aggressive conditions decreases to 2 μm. Our conclusion is consistent with empirical data based on microtube and immersion data published by academics and other researchers in the industry.
Sartorius Stedim Biotech has developed and validated a robust bacterial aerosol challenge test to further support its integrity testing program. We believe that this test will provide the most useful and relevant data available for correlating microbial ingress to a physical integrity test. Holes of 2–100 μm were laser-drilled into samples of S70 and S80 films. Those samples then were exposed to aerosolized Bacillus atrophaeus spores with a size distribution of 0.2–0.3 μm and a concentration of 106 cfu/cm2 for 3 hours. Tryptone soya agar (TSA) medium from the downstream side of the film then was incubated for 7 days at 30–35 °C. No colonies were observed from films containing defects of 40 μm or less for either film. That result falls in line with existing published data from aerosol tests performed on 20-μm to 50-μm diameter microtubes.
We used that analysis to guide development of a three-stage single-use system integrity-testing strategy. Based on the analysis above, we defined a detection limit of 2 μm for bags that our customers intend to use in critical applications, including liquid shipping. To reach that limit, we implemented an integrity test based on helium detection. That test is performed on final assemblies before their sterilization and shipment. Before assembly, however, all bag chambers are subjected to an ASTM F2095 pressure-decay test that can detect defects in the range 40–100 μm as part of our process control strategy (Figure 1).
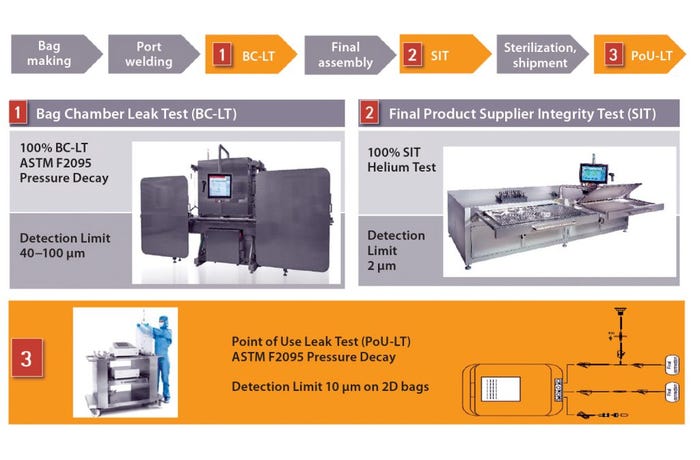
Figure 1: Integrity testing along the entire life cycle improves process integrity
Biomanufacturers can ensure that no defects have been introduced into 2D and 3D bags during shipping and handling with our point-of-use leak test. It is the third test in the single-use system life cycle. The leak tester will detect defects as small as 10 μm in 2D bags, which is smaller than the 15-μm MALL that allows leaks and microbial ingress in storage bags used under controlled conditions.
We are working to correlate those detection limits to aerosol bacterial challenge tests, which are more representative of actual process conditions. Our patented aerosol bacterial challenge test allows more tests to be performed and thus provides for greater statistical validity.
Robust Single-Use Systems
Figure 2: Robustness and closure integrity by design
The ability to identify when defects exist within single-use systems is, of course, only part of the problem. Suppliers of single-use technologies must take steps to reduce the number of defects in the first place. Designing robust single-use bags is the foundation for providing assurance of container– closure integrity (Figure 2). Sartorius Stedim Biotech has applied quality by design (QbD) principals to ensure that its bags will be robust across the manufacturing design space. The bags have been validated using the most stringent standards such as ASTM D4169 for shipping. And the company has introduced technology such as self-deploying bags to prevent mishandling and thereby reduce the risk of bag failures.
The risk of defects in films, seals, welds, and connections are reduced by stringent process controls. Sartorius Stedim Biotech carefully controls many process parameters, such as film-extrusion temperature and speed, weld and seal temperatures, and times. Quality control protocols confirm the absence of leaks in all single-use systems. For instance, the company routinely performs an ISO 15747 immersion bacterial-challenge test to confirm the microbial integrity of representative final products. A detailed understanding of failure modes that cause leaks from single-use systems helps focus continuous-improvement efforts on further reducing the number of bag defects.
Our detailed records are continuously updated, showing that of the million bags Sartorius Stedim Biotech produces each year, ~400 have defective bag chambers, most of which are identified before leaving the production facilities. Only 20 of those million bags have defects that lead to leaks at customer site — however, that is still too many. Our target for continuous quality improvement is to eliminate all leaks caused by production failures at Sartorius Stedim Biotech locations. A similar number of defects come from damage that occurs because of bag transportation or handling problems. Again, we see it as our responsibility to help reduce the number of such defects to zero. Point-of-use leak testing will help identify single-use systems that are not integral, but the defects themselves can be reduced by thorough package and liquid-shipping training, self-deploying technologies, and operator expertise.
For the benefits of single-use technologies to be realized fully within commercial biomanufacturing facilities, the industry must takes steps to understand container–closure integrity fully. We continue to conduct research into the links between bag defects, liquid leaks, and microbial ingress. This research is helping Sartorius Stedim Biotech to develop sophisticated integrity-testing strategies at its production facilities and for biopharmaceutical production sites. As part of these strategies, we will correlate our integrity tests to bacterial aerosol challenge tests. The ability to identify potential leaks before use is important for biomanufacturers; however, Sartorius Stedim Biotech is working hard on continuous quality improvements to further improve the robustness of its single-use systems. •
Carole Langlois is senior FMT product manager at Sartorius Stedim Biotech FMT. Marc Hogreve is senior engineer in integrity testing solutions at Sartorius Stedim Biotech.
You May Also Like





