- Sponsored Content
- Manufacturing
Ensuring Leak-Free Performance During Tissue Container Shipping Utilizing ASTM D4991
November 17, 2022
Date: Nov 17, 2022
Duration: 20 Min
Sponsored by Savillex
This webcast features: Eric Isberg, Vice President, Life Sciences, Savillex.
Companies in the fields of tissue engineering and cell therapy find that when standard, off-the-shelf labware containers are used to transport therapies, the container seal can fail during shipment. For a live tissue or cell sample being shipped to a hospital or clinic as part of a treatment, this can be a catastrophic failure that can lead to damaged samples, surgical delays, and poor patient outcomes.
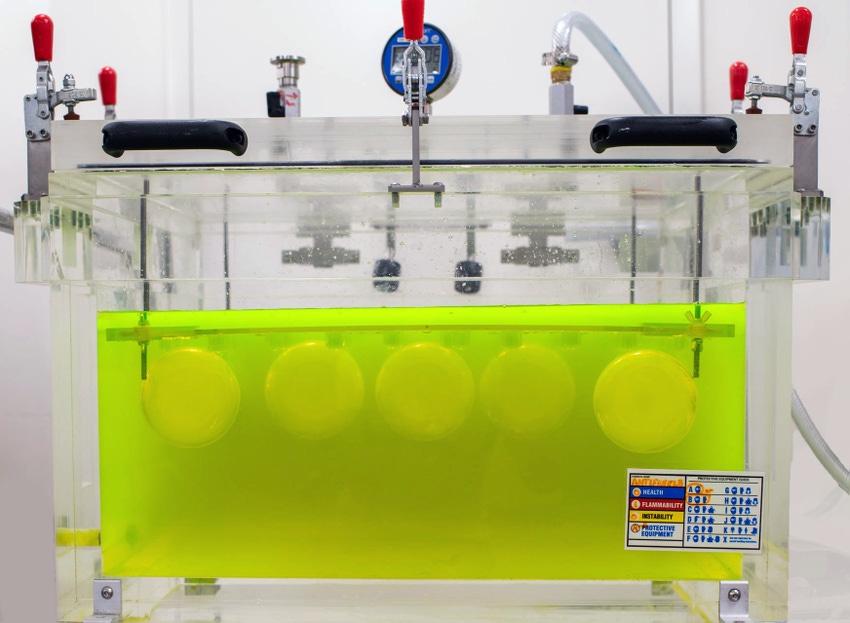
When selecting containers for critical CGT applications, those that have been tested to ASTM shipping test standards are strongly preferred. The ASTM D4991-07(2015) Standard Test Method for Leakage Testing of Empty Rigid Containers by Vacuum Method is a standard test method which covers the testing of empty rigid containers under differential pressure conditions like those which can occur during air transport.
It is an important test for companies considering using bottles, jars, vials, or other containers for critical shipping applications, particularly when maintaining sterility is required.
This webinar describes the testing of Purillex® bottles and jars by an independent lab to meet the requirements of ASTM D4991.
Key takeaways:
Understand the impact of container failure on engineered live tissues.
Learn details of the ASTM D4991 test method for containers used to ship live tissues.
View container features and usage methods that can ensure passing D4991 testing.
Just fill out the form below to view the recorded webcast now.
About the Author(s)
You May Also Like





