- Sponsored Content
- Chromatography
µPAC™ Microchip Chromatography: Better By Design
Sponsored by PharmaFluidics
 The boundaries of technology can be pushed significantly when insights from different fields reinforce each other. Based on in silico simulations demonstrating the importance of order on the efficiency of chromatographic separations, PharmaFluidics has combined expertise from the analytical chromatography and semiconductor chip manufacturing industries to create a new type of nanoscale liquid chromatography (LC) column.
The boundaries of technology can be pushed significantly when insights from different fields reinforce each other. Based on in silico simulations demonstrating the importance of order on the efficiency of chromatographic separations, PharmaFluidics has combined expertise from the analytical chromatography and semiconductor chip manufacturing industries to create a new type of nanoscale liquid chromatography (LC) column.
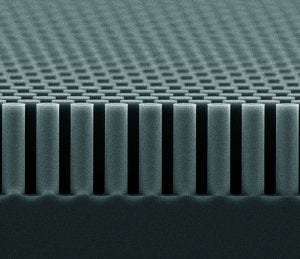 Conventional LC columns contain randomly packed beads as a stationary phase. By contrast, PharmaFluidics uses a lithographic etching process to create a perfectly ordered separation bed on a silicon chip. This new type of microchip-based chromatography column is a micro Pillar Array Column (µPAC) device.
Conventional LC columns contain randomly packed beads as a stationary phase. By contrast, PharmaFluidics uses a lithographic etching process to create a perfectly ordered separation bed on a silicon chip. This new type of microchip-based chromatography column is a micro Pillar Array Column (µPAC) device.
A low “on-column” dispersion comes with the perfect order, which virtually eliminates axial peak dispersion. That provides for higher column-plate numbers with sharper peaks and higher concentration of compounds than would be otherwise possible. The freestanding nature of the pillars also allows for much lower backpressure than other methods, allowing the use of very long columns. These exceptional properties result in excellent chromatographic performance with high resolution and sensitivity. This new approach significantly improves LC analysis for complex mixtures of biological samples.
 Furthermore, the micromachined separation bed in the µPAC chips forms a rigid structure that is perfectly symmetrical, allowing bidirectional operation without risk of stationary-phase compression, column deterioration, or stationary-phase leakage. Finally, all µPAC microchips are identical because of their lithographic production process. Therefore, a µPAC column serves as a robust, high-resolution separation tool.
Furthermore, the micromachined separation bed in the µPAC chips forms a rigid structure that is perfectly symmetrical, allowing bidirectional operation without risk of stationary-phase compression, column deterioration, or stationary-phase leakage. Finally, all µPAC microchips are identical because of their lithographic production process. Therefore, a µPAC column serves as a robust, high-resolution separation tool.
A New LC Approach for Biomarker Discovery
Because of those advantages, µPAC chips are ideally suited for applications in fields that need to analyze small volumes of highly complex samples. Even tiny differences among compounds are sufficient to obtain chromatographic separation. Furthermore, high sensitivity is ensured because the compounds remain concentrated. The µPAC system thus allows detection of individual compounds across wide dynamic concentration ranges.
That can be useful in identifying new target molecules for drug discovery or in finding new biomarkers for diagnosing and following up on disease progression. The µPAC system will support new discoveries in research on cancer, cardiovascular diseases, and hereditary or chronic conditions such as Alzheimer’s disease.
For example, the system can be applied to bottom-up proteomics for limited sample amounts. One study has shown that combining µPAC columns with high-resolution mass spectrometry (MS) is suitable for proteomics experiments in which the sample amount is limited to a small number of cells (1). Injecting a dilution series of 1 µg, 100 ng, and 10 ng of HeLa cell digest has provided high protein coverage from both the highest (>5,400 protein groups) and lowest (about 50 cells, >3,000 protein groups) concentrations. As a consequence, µPAC columns present a new tool for limited-sample proteomics.
Innovative Approach to MAb and ADC Characterization
Another growing market where µPAC chips will have a significant impact is in the characterization of monoclonal antibodies (MAbs). By contrast with small-molecule drugs, MAbs are large molecules (~150 kDa) and heterogeneous in nature. Small modifications such as glycosylations or deamidations can have a tremendous influence on the therapeutic efficacy of a MAb. In antibody–drug conjugates (ADCs), the antibodies are used to guide cytotoxic drugs to specific cells. Conjugation can happen on different sites, creating different isomers, which further increases complexity of the drug. Analytical capabilities should be able to distinguish between all those small modifications.
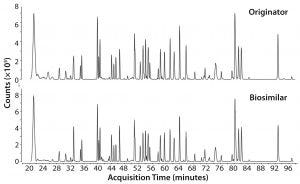
Figure 1: µPAC-MS total compound chromatograms of a Herceptin originator and candidate biosimilar tryptic digest.
In addition, as patents on the first biopharmaceuticals are expiring, an explosion of biosimilar activity has taken place. Creation of biosimilar products is facing major hurdles, however. By contrast with generic versions of small molecules, exact copies of recombinant MAbs cannot be produced because of differences in cell clones and biomanufacturing processes used. Thus, regulatory agencies evaluate biosimilars based on their level of similarity rather than exact replication of an original comparator drug. For development and regulatory filing of biosimilars, precise analytical data substitutes for extensive additional clinical studies.
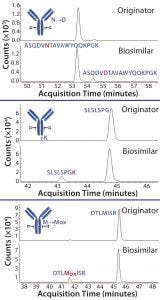
Figure 2: Extracted chromatograms of selected modified peptides measured in Herceptin originator and candidate biosimilar; asparagine deamidation, lysine truncation, and methionine oxidation are elevated in the biosimilar compared to the originator. Locations of the modifications are highlighted in the antibody structure.
The value of µPAC columns in characterizing MAbs was demonstrated recently at the Research Institute of Chromatography in Kortrijk, Belgium (2–4). Using Herceptin (trastuzumab) and a candidate biosimilar, the prevalence and location of posttranslational modifications could be determined reliably (Figures 1 and 2) (2). In an analysis of the Kadcyla ADC, µPAC resolving power enabled an in-depth study of conjugation sites and achieved the separation of isomeric conjugated peptides (3). Finally, when comparing Remicade (infliximab) with a candidate biosimilar, point mutations could be identified (4). Because regulatory authorities consider an identical primary sequence primordial to similarity, that biosimilar candidate would be ruled out for further development. Being able to do that in early stage development, or otherwise confirming similarity in detail, is of the greatest importance both for biopharmaceutical companies and regulatory authorities.
A Unique Technology
For screening complex biological samples to discover rare molecules — and when searching for tiny differences to characterize MAbs and ADCs — µPAC columns belong in every analytical laboratory. PharmaFluidics μPAC technology is unique in its kind: built on the precise micromachining of designed pillar-array separation beds into silicon. Because of its smart design, data productivity and robustness are no longer a concern in nanoscale LC.
References
1 Op de Beeck J, et al. Poster: A Novel Nanoflow LCMS Limited Sample Proteomics Approach Using Micro Pillar Array Columns (μPAC™). Thermo Fisher Scientific Inc. and PharmaFluidics NV, 2017; www.pharmafluidics.com/wp-content/uploads/ThermoFischerScientific_PharmaFluidics_HUPO2017.pdf.
2 Sandra K, et al. Application Note: Monoclonal Antibody Peptide Mapping Using Micro Pillar Array Columns Combined with Mass Spectrometry (μPAC™-MS): Part 1, Herceptin® Originator and Candidate Biosimilar. PharmaFluidics NV: Ghent, Belgium, 2017; www.pharmafluidics.com/monoclonal-antibody-peptide-mapping-herceptine-originator-and-candidate-biosimilar.
3 Sandra K, et al. Application Note: Characterizing Antibody-Drug Conjugates Using Micro Pillar Array Columns Combined with Mass Spectrometry (μPAC™-MS). PharmaFluidics NV: Ghent, Belgium, 2017; www.pharmafluidics.com/characterizing-antibody-drug-conjugates-using-micropac-ms.
4 Sandra K. Application Note: Monoclonal Antibody Peptide Mapping Using Micro Pillar Array Columns Combined with Mass Spectrometry (μPAC™-MS): Part 2, Remicade® Originator and Candidate Biosimilar. PharmaFluidics NV: Ghent, Belgium, 2017; www.pharmafluidics.com/remicade-originator-and-candidate-biosimilar.
Katrien Vanhonacker is vice president of business development and sales, and Dr. Jeff Op de Beeck is application development manager at PharmaFluidics, Technologiepark-Zwijnaarde 3, B-9052 Ghent (Zwijnaarde), Belgium; 32-9-241-5657; [email protected], [email protected]; www.pharmafluidics. com. µPAC is a trademark of PharmaFluidics.
You May Also Like





