Scientific and Technological Advancements in Applications of Single-Use Technology: A Conference Report

The “Single-Use Technology: Scientific and Technological Advancements” conference took place last autumn in the Wasatch Mountains of Utah. (WWW.ISTOCKPHOTO.COM)
Single-use technology (SUT) has been used increasingly both in clinical and commercial biomanufacturing (1). Proven major advantages include relatively low capital investment, elimination of batch-to-batch cross contamination and reuse cleaning validation efforts, flexibility in manufacturing, and shortened product lifecycles. However, some challenges and barriers to implementation remain: Consumables costs are increasing. Specific regulatory guidance is lacking, as is component interchangeability and standardization. And few if any leak-proof components/systems are available. International groups and associations focused on setting best practices and standards to ensure reliable and risk-free biomanufacturing include the American Society for Testing and Materials (ASTM), the BioPhorum Operations Group (BPOG), the Bio-Process Systems Alliance (BPSA), the Parenteral Drug Association (PDA), the US Pharmacopeial Convention (USP), and the Extractables and Leachables Safety Information Exchange (ELSIE). Some companies have implemented SUT successfully in end-to-end commercial monoclonal antibody (MAb) drug-substance manufacturing (2), and many others are using SUT in clinical processes.
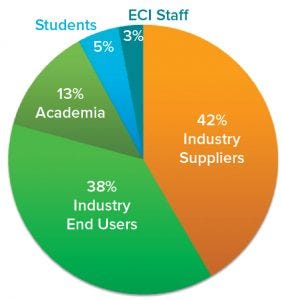
Figure 1: SUT III participant statistics by type of institution represented (97 participants in total)
A common interest in SUT brings industry and academic experts together at the ECI SUT Conference series. The third edition — titled “SUT III: Scientific and Technological Advancements” — took place in Snowbird, UT, on 23–26 September 2018. As conference cochairs, we organized and hosted the meeting with support from the staff of Engineering Conferences International (ECI). Some 100 professionals working or interested in the single-use field were invited, attended, and actively participated in all activities (Figure 1).
Stefan Schmidt (BioAtrium AG) delivered the first keynote, titled “Process Intensification in Biomanufacturing Driven By Advances in Single-Use Technologies.” He stressed that opportunities abound in development of novel, disruptive SUTs to intensify drug-substance biomanufacturing processes, including both upstream and downstream unit operations. In upstream production, the highest efficiencies are achieved by improving seed/inoculation train processes and eliminating process steps. In downstream separation and purification, the biggest benefit can be realized by combining operations such as single-pass tangential-flow filtration (SPTFF) and chromatography. Personalized medicine can benefit significantly from intensified processes designed using disposable equipment.
David W. Grainger (distinguished professor from the University of Utah) presented another well-received keynote entitled “Polymer Interfaces and Biopharmaceuticals: Chemistry, Design and Challenges.” He explained how cells and proteins respond to outer atomic layers or the first few monolayers of a surface (about 3 nm). All proteins have some interfacial activity, stability, and affinity on surfaces. Scientific insights about the interaction of polymeric surfaces with biologics helped the audience understand the most probable reaction mechanisms. That is especially valuable because the effect on cells/proteins of gamma (γ) irradiated polymers (often used in SUT) still presents a challenge in implementing SUT for biomanufacturing.
Session 1
Polymers in New Biopharmaceutical Applications: Cochairs Magali Barbaroux (Sartorius Stedim Biotech) and Sheryl Kane (Amgen) led the session and follow-up discussion initially focused on existing technologies and further exploring how to push the boundaries of current SUT capabilities toward developing novel materials. Some themes that emerged from this session included the importance of risk assessments and an awareness that polymers can affect bioprocessing in both positive ways (by enabling new technologies) and negative ways (through material degradation, leachables, and chemical incompatibility).
Todd Andrews (Colder Products Corporation) began the session with an overview of the design process for development of a new connector, focusing on user requirements, manufacturing, and validation. He highlighted the importance of materials selection to meet user requirements regarding sterilization and chemical compatibility, as well as manufacturing implications concerning moldability. Panel discussion emphasized the need for a standard or universal connector to work with bags from different vendors and identified a need to limit the risk of incorrect connections between different lines. Possible solutions include implementation of color-coded lines.
Nelly Montenay (Sartorius Stedim Biotech) discussed the design of an accelerated aging test plan. She emphasized understanding the complete lifecycle of raw materials and analyzing risks to ensure that testing will capture problems adequately for actual material use while minimizing chances of introducing failure modes that would not occur in use. Discussions focused on whether the standard Arrhenius model for accelerated aging accurately predicts material behavior and whether real-time aging studies should be performed to confirm accelerated aging results.
Anu Vaidya (Biogen) shared her experience in adapting single-use systems for a solvent-based small-molecule synthesis process to make therapeutic antisense oligonucleotides. She highlighted material challenges such as chemical compatibility and leachables; validation challenges related to operating outside supplier-validated conditions; and sourcing challenges such as second sourcing and when materials are not intended for pharmaceutical industry use. Despite those issues, Biogen was able to develop a primarily single-use process to produce antisense oligonucleotides.
Finally, Professor Jules Magda (University of Utah) introduced the possibility of using smart hydrogels for sensing applications in the biotechnology industry. He advocated for the use of synthetic rather than biological molecular-recognition elements to provide analyte-specific functionality with superior stability, sterilization compatibility, and tunable binding capacity. The panel emphasized the need for reversible binding and challenged assumptions about user requirements for sensors (e.g., response times for applications such as bioreactors).
Session 2
Interaction of Polymers with Bioprocess Fluids and Bioproducts (Including Extractables and Leachables): Cochairs Xueyuan Wang (Roche/Genentech) and Chor Sing Tan (GE Healthcare) led this session. Xueyuan opened with a statement about the importance of understanding interactions between polymers and proteins when SUT is used during formulation, storage, freezing, and transportation of biopharmaceutical solutions. To date, very few studies of such use have been published, with most focusing on interactions between cells and polymers.
Samuel Dorey (Sartorius Stedim Biotech) presented his work on the impact of γ irradiation on polymers in a multilayer film by studying diffusion and release (spontaneous migration of molecules from a single-use bag). The film in his study was made from two layers of ethylene-vinyl acetate (EVA) surrounding a layer of ethylene vinyl alcohol (EVOH). Both nonspecific — total organic carbon (TOC), pH, and conductivity — and specific (ion-exchange chromatography and spectroscopy) analytical methods were used in this study. Principal component analysis (PCA) was used to interpret the results. This study showed a clear trend in growing influence of the γ-irradiation dose (≤270 kGy) on the multilayer film. Direct proportionality was observed between the quantity of released carboxylic acids and measured TOC. It was found that carboxylic acids represented a lower percentage of the TOC amount measured after long storage times than after short-term storage. That suggests the presence of carbon not related to carboxylic acids with long-aging extraction and at high gamma doses. The ion-exchange chromatography identified mainly formic and acetic acids as ionic leaching species from the multilayer film, and storage-time effects were observed. At least 80% of ions are extracted within 30 days. This presentation showcased the effective use of multiple analytical methods to discern (ionic) leaching species from polymer films and thus determine the stability and shelf life of single-use bags made from one multilayer polymer film under accelerated test conditions.
During the panel discussion, Dorey was joined by Weibing Ding (GlaxoSmithKline), Christian Julian (Meissner Filtration), and Ekta Mahajan (Genentech, a member of the Roche Group). Dorey explained that his study also included a polyethylene (PE) multilayer film that released less TOC and 10× less acid than did the EVA-based film.
BPOG’s standardized extractables testing protocol was designed to mitigate patient, product, and regulatory risks potentially faced by SUT end users (3). In 2017, the USP published a draft chapter <665> for an extractables standard test method. Both documents have been well thought through, stemmed from numerous industry discussions, and were subject to several scientific assessments. Attendees discussed the differences between them. The BPOG protocol recommends six solvents and three to four time points per component; USP requires three solvents with single time points. But the solvents and time points basically are aligned between the two documents. USP <665> provides minimum requirements that address the most commonly encountered bio/pharmaceutical situations and generally can be considered to be a subset of BPOG’s protocol.
Session 3
Sensors and Their Integration with Single-Use Technology: Cochairs Gernot John (PreSens Precision Sensing) and Prashant Tathireddy (Applied Biosensors) led this session. Alongside the adoption of new technologies, the need for reliable sensors also has grown. Development of new sensing technologies is imperative for biomanufacturing to enable advanced process control, closed-loop processes, and the adoption of quality by design (QbD) methodologies. This session was designed for discussing the status of existing technologies and to present new ideas.
The first speaker, Jordan Cobia (Thermo Fisher Scientific), discussed the importance of using an automated single-use foam-control strategy with single-use bioreactors (SUBs). Foam buildup in bioreactors causes problems ranging from unfavorable metabolic conditions for cells to potential failure of a bioprocess container. The efficacy was demonstrated of coupling a single-use foam probe with antifoam addition automated by an integrated DeltaV distributed control system (Emerson US). Aggressive fed-batch operating parameters showed that the probe greatly reduced the amount of foam present and led to fewer required antifoam additions. The work demonstrated
foam probe selection and integration into a Thermo Scientific S.U.B. bioprocess container
optimal control parameters of antifoam addition using real-time feedback from the foam probe and integrated controller.
A presentation by Nicholas Frazier (Applied Biosensors) focused on a novel sensing technology based on responsive hydrogels for monitoring antibody production. This talk detailed the technical development and performance testing of immunoglobulin G (IgG) sensors. Analytical techniques were used to characterize and select aptamers with appropriate binding affinity to IgG before their integration into the hydrogels. Results showed that the IgG-sensitive hydrogel is stable to autoclaving and γ irradiation procedures and that it responds to increasing and decreasing concentrations of IgG in different media.
Nick Rummel (Genentech/Roche) reported on evaluation of new pH sensors for upstream single-use applications. Prototype sensors from four different vendors were evaluated for their ability to replicate the robustness and reliability of multiuse sensors. A series of experiments compared the performance of these sensors with that of a traditional glass, multiuse pH probe. Data from these experiments provided a basis for assessing the performance of each sensor and determining whether it is viable for implementation in upstream single-use applications both in development and good manufacturing practice (GMP) manufacturing.
Finally, Patrick Sagmeister (Exputec) presented a new sensing technology based on data analytics called soft sensors for single-use bioprocessing. He demonstrated that information mining and process analysis based on a combination of mechanistic models and statistical tools can be used to control processes both efficiently and scalably.
Session 4
Single-Use Advantages in Continuous and Connected Processing: Cochairs Ekta Mahajan (Roche/Genentech) and Ruben Carbonell (North Carolina State University) led this session. It is evident from results presented at this conference that great advances are being made toward strategies and applications of continuous and connected manufacturing concepts, all facilitated by single-use bioreactors and downstream operations.
Robert Kottmeier (Pfizer) reported on pilot-scale implementation of a high-intensity, integrated process for making MAbs with single-use technology. The cell culture process involved a single-use bioreactor connected to a tangential-flow filtration (TFF) device for perfusion operation. Two protein A columns were used for product capture directly from the bioreactor, with no hold steps or surge vessels. Their elution fed directly into a continuous viral-inactivation step facilitated with a custom-designed 3D-printed flow chamber that provided the necessary low-pH residence time for viral inactivation. Changes in pH upstream and downstream of that unit operation were made by directly adding acid or base to the product feed stream just before and after the flow chamber. Static mixers ensured rapid blending of both process and buffer streams. A disposable anion-exchange (AEX) column and SPTFF unit completed the downstream process. The pilot process yielded 2.5 kg of product over a 16-day campaign and will be implemented for manufacturing phase 1 clinical materials. Single-use devices implemented in this process included probes for pH and dissolved oxygen (DO), a camera for foam control, and an autosampling device for process analytical technology (PAT). The combination of continuous processing and single-use devices in this case holds great promise for reducing costs and footprints while improving quality control over what is achievable with fed-batch processes.
Stefan Junne (TU Berlin) gave an eloquent argument for the value of combining single-use devices and continuous processing concepts in microbial fermentation. He mentioned several problems that must be solved for them to work: large cell densities, oxygen and CO2 transfer rates, and process monitoring and control for extended culture periods. Recent results have shown that a Cell-tainer system (a 14-L bag reactor with 2-D rocking movement) could provide kLa values similar to those obtained with stirred tank reactors but without the stirrer that can cause cell damage. With bag holder modification, it was possible to run this system with 500 mL, 10 L, and 150 L of medium to facilitate scale-up. Junne showed that the reactor’s motion could affect the morphology of precipitates formed during fermentation.
Ben Madsen (Thermo Fisher Scientific) showed that a modified gas-sparger design and increased impeller sizes in a Thermo Fisher S.U.B. system could increase the reactor’s kLa value 5× from about 10/hour to 50/hour. Results indicated that Chinese hamster ovary (CHO) cell densities up to 270,000,000 cells/mL could be obtained with the modified reactor. Although such very high cell densities could be detrimental to product quality, the results do indicate that significant improvements in S.U.B. operation can come from relatively modest changes in bioreactor design.
Alex Chatel (Univercells) demonstrated the results of a highly innovative, low-footprint, intensified SUT platform for production of viral vaccines. The objective was to lower the production cost for attenuated Sabin polio vaccines to about US$0.15/dose using a small-footprint process that could be deployed easily to different locales around the world where such a vaccine might be needed. A novel, packed-column bioreactor with nonwoven fabric to support adherent cells was operated in-line with an ultrafiltration TFF cartridge for perfusion. Cell culture fluid was clarified by size-exclusion chromatography and purified with a hydrogel-based multimodal cation-exchange (CEX) column followed by a viral inactivation step. Overall product recovery was 40%. A 600-m2, 50-L fixed-bed reactor produced 2.4 million doses per batch, the same amount of virus as 7,000 roller bottles or a 1,000-L stirred-tank single-use bioreactor with microbeads. This new approach holds great promise for production of biosimilars, for which price point and ease of technology transfer are key objectives.
Session 4 presentations led to a vigorous panel discussion that touched on many topics concerning the technical details of reported studies as well as higher-level strategic issues with continuous processes, SU devices, and their role in the future of biomanufacturing. Two major themes of discussion were how to implement PAT approaches during continuous processes in general and the lack of in-line and on-line technologies to measure products’ critical quality attributes (CQAs) and process-control variables. Regulatory issues also were discussed, including how to define a continuous process batch, what to do in the event of deviations during continuous production, and how to plan for potential failures at different points in continuous operations.
Design of single-use bioreactors and downstream devices also received great attention, as did the idea of monitoring their quality before use and testing for potential failures. It is clear from the presentations and panel discussions in this session that continuous, connected, and integrated processes are the future of biomanufacturing and that single-use devices will play key roles in enabling the desired features of such processes. In the years to come, continued efforts on the part of drug manufacturers, vendors/suppliers, and academics will focus on development of new products and processes that can fill gaps associated with PAT, upstream and downstream operations, drug-product development, and regulatory issues. Ultimately, that will help make biopharmaceutical manufacturing with these new processes a reality.
Session 5
Single-Use Adoption for Cell and Gene Therapy Applications: Cochairs Tiffany Hood (MilliporeSigma) and Margarida Serra (Instituto de Biologia Experimental e Tecnológica, IBET) led this session focused on advances made in SUT for cell and gene therapies. Speakers offered examples and case studies showing closed automated solutions that have enabled commercial manufacture, ensured consistent quality, and/or helped companies meet commercial demand.
Marc Hogreve (Sartorius Stedim Biotech) opened the session by describing the increased need for container–closure integrity for cell and gene therapy applications. His case studies illustrated development of deterministic integrity-testing technologies, with sensitivities correlated to microbial ingress and liquid leaks under process conditions.
Gary Lye (University College London) and Margarida Serra next discussed how current single-use technologies can be applied and optimized for cell therapy manufacturing. In particular, he reviewed the use of small-scale models such as microbioreactors in process development for cost-efficient expansion of two types of human adult stem cells (human T-cells and mesenchymal stem cells, hMSCs). Lye’s team evaluated the effects of critical process parameters (microcarrier type, medium formulation, and stirring rate) on stem cell expansion. Serra showed how optimized upstream processes for hMSC expansion can be scaled up in single-use bioreactors and then integrated with downstream processing steps such as microcarrier removal, volume reduction, and washing. Alongside the standard quality assays for evaluating hMSC CQAs, she presented a proteomics workflow based on mass spectrometry tools for use in characterizing the impact of processing.
Matt Marsh (Hitachi Chemical Advanced Therapeutic Solutions) closed the session by describing challenges and technology gaps in commercial-scale cell therapy manufacturing. He provided a case study of a single-use counterflow centrifugation (CFC) device developed with Invetech for increased cell isolation and harvesting efficiency.
This session highlighted a need for high-quality, closed, SUTs to reduce production times and costs in cell and gene therapy manufacturing. One big challenge identified was that few single-use systems/components were developed specifically for these applications, which cannot depend on traditional sterile barriers or economies of scale. Attendees also discussed a need for robust and rapid analytical tools in product characterization and process understanding.
Session 6
Single-Use Performance: Cochairs Regine Eibl-Schindler (Zurich University of Applied Sciences, ZHAW) and Stefan Junne (TU Berlin) led this session. A new generation of single-use devices is making it possible for bioprocess engineers to design complete bioproduction processes. The biopharmaceutical industry has increased confidence in single-use technologies today, although the devices still have limitations. Recent equipment developments have been driven by the requirements of end users, their processes, and final products: e.g., advances in film technologies, bioreactor design, sensor systems, and automation. Robustness and handling of single-use systems have been improved. The four talks in this session highlighted several remaining challenges.
Diego Schmidhalter (Lonza) discussed benefits and drawbacks of single-use devices in antibody–drug conjugate (ADC) manufacturing. ADC production requires handling of components with cytostatic and cytotoxic characteristics. Occupational safety, health aspects, and crosscontamination prevention require special attention. The main advantages of single-use devices for ADC manufacturing include reduced exposure risk for process operators, minimized toxic waste streams from cleaning processes, and decreased investment costs, particularly in clinical manufacturing. Major drawbacks of single-use equipment remain the risk of leachables and leakage during key unit operations. Those risks are manageable with risk assessment and diligent selection of single-use equipment. The speaker was optimistic that existing limitations will be resolved and that technology solutions from ADC production will pave the way for application of single-use devices to other areas of drug manufacturing.
Klaus Wormuth (Sartorius Stedim Biotech) described risk assessment of particulate contamination in biopharmaceutical processes considering product quantity, quality, and patient safety. He explained cost factors for risk assessment and strategies for implementation in different unit operations, and he showed how filtration and purification steps can reduce the risk of contamination. Standards for purification exist only for final drug products; however, particulates could have secondary effects during processing. Leachable chemicals from intrinsic particles are the same as those from the devices themselves, and the increase in surface area due to those particles is insignificant. Another question remains as to whether particulate contamination from single-use systems could increase protein aggregation. Wormuth introduced a holistic approach for reduction of particulates and highlighted remaining challenges for process steps downstream of final filtration, which are closest to the administration to the patient: final filling and aseptic processing of vaccines and gene/cell therapies. (See this month’s “Supplier Side” article for more from Wormuth and his colleagues on this topic.)
Jason Brown (Thermo Fisher Scientific) compared single-use and stainless-steel bioreactors for kLa values and addressed their suitability for microbial fermentation. The share of single-use bioreactors used for aerobic cultivation of microbes is increasing, which raises the question of how well these systems can meet the requirements for such cultures. Two challenging parameters are gas mass transfer and power input. Brown demonstrated kLa studies and aerobic cultivations with ≤2 vvm gas flow in single-use bioreactors with 6-L to 300-L working volumes. The reactors are designed to meet the performance requirements of dense, rapidly growing microbial cultures while offering benefits of rapid process setup, reduced contamination risk, and high production quality. Results demonstrated that opportunities do exist for beneficial use of single-use systems in microbial processes.
Finally, Bill Whitford (GE Healthcare) presented a lifecycle assessment approach for single-use materials. He showed results of recent studies that clearly indicate, in most installations, a lower environmental impact from single-use technologies than from traditional stainless-steel equipment. Impacts related to disposal of single-use equipment at the end of its life often are debated, but in the context of cradle-to-grave lifecycles of biomanufacturing, they actually are negligible. Processes based on reusable equipment have more impact on energy consumption originating from clean-in-place/steam-in-place (CIP/SIP) and water for injection (WFI) use. However, Whitford showed that packaging of single-use systems for safe transport should not be underestimated, especially with growing scales. Optimization of and reduction in packaging materials (e.g., overbags, cartons, and protective foam) help reduce material turnover, storage, and discard while increasing the sustainability of single-use manufacturing.
Workshops
Two workshops were chaired by Mike Goodwin (Thermo Fisher Scientific), Arthi Narayanan (Genentech/Roche), Razvan Miclea (Amgen), and Ernie Jenness (MilliporeSigma).
Training challenges include identifying the specific needs of different learners, setting training objectives, time constraints in preparation and participation for learning events, and the need to maintain continuous learning programs beyond such events or courses. Suggested training topics include materials of construction, unit operation processing, installation and handling of bags, making proper connections, and disposal of used equipment and components. Training methods can be instructor-led or computer-based “e-learning” provided by either internal trainers or external organizations. Internationally, providers include the Biomanufacturing Education and Training Center at Worcester Polytechnic Institute (WPI), the Jefferson Institute for Bioprocessing (JIB), NC State University’s Biomanufacturing Training and Education Center (BTEC), the National Center for Therapeutics Manufacturing (MCTM), Texas A&M’s Engineering Experiment Station, the National Institute for Bioprocessing Research and Training (NIBRT), other universities and online organizations, and technology suppliers.
When single-use drug product final fill assemblies are implemented along with an isolator, how can end users prevent ingress of vapor-phase hydrogen peroxide (VPHP) into drug-product pathways? Two modes of operation can be applied: The most common approach among end users is to introduce SUT into an isolator through a rapid-transfer port at the end of the aeration phase, when residual levels of hydrogen peroxide are below a set threshold, to prevent drug-product degradation. The second mechanism is to use materials of construction that are resistant to hydrogen peroxide permeation.
What is the state of control for zero particulate defects in final filling assemblies downstream of sterile barriers (e.g., sterile filters)? This is a major topic of interest for both SUT end users and manufacturers. The risk associated with subvisible particles is low and fairly well understood. Nearly all suppliers have developed capabilities to test their products using standardized methods in agreement with USP <788>. However, no standard methodology has been agreed upon for extracting, isolating, detecting, and reporting visible particles. Without standardization, it is difficult for SUT manufacturers and end users to prove that a given manufacturing step will yield “essentially no” visible particles. It was recommended that the industry move toward standardization in definition and methodology to extract, isolate, detect, and report visible particles. And a similar approach was recommended for standardizing extractables studies across the industry (e.g., BPOG extractables protocol, USP <665>) as the best option for moving forward.
Looking to the Future
The SUT future is bright, with increasing implementations of single-use systems in both clinical and commercial biomanufacturing. Innovations in sensors and polymers are needed and in process. It was clear from these conference discussions that interest is growing in the implementation of SUT for intensified processes, continuous processes, and relatively new applications such as cell and gene therapy processing. The whole SUT industry needs to collaborate toward standardization (following the approach adopted for an extractables standardized protocol). Effort must be made toward better understanding of the interactions between polymers and cells/proteins. New and in-depth studies for improving polymer films and assembly manufacturing technologies would ensure the supply of robust SUT with consistent quality attributes for biomanufacturing — ultimately to serve patients better than ever before.
The fourth edition of this conference series is scheduled for May 2020. Once again, it will be intended to inspire the community with adventurous presentations from experts in the field, case studies and lessons learned, and examples of solutions from other industries as well as innovative proposals to tackle emerging areas. Detailed conference information will be published online at http://www.engconf.org/conferences/biotechnology.
References
1 Langer ES, et al. 15th Annual Report and Survey Biomanufacturing. BioPlan Associates, Inc.: Rockville, MD, April 2018.
2 Ding W. Single-Use Systems and Their Use in Biopharmaceutical Manufacturing: Benefits and Challenges. USP-SUS China Workshop, August 2018: Suzhou, China.
3 Ding W, et al. Standardized Extractables Testing Protocol for Single-Use Systems in Biomanufacturing. Pharm. Eng. November/December 2014: 74–85.
Acknowledgments
We thank Magali Barbaroux, Sheryl Kane, Xueyuan Wang, Chor Sing Tan, Gernot John, Prashant Tathireddy, Ekta Mahajan, Ruben Carbonell, Tiffany Hood, Margarida Serra, Regine Eibl-Schindler, Stefan Junne, Mike Goodwin, Arthi Narayanan, Razvan Miclea, and Ernie Jenness for their contributions to this manuscript — and all the speakers and other conference participants for their active engagement. We also thank ECI staff and Beth Junker for support and guidance that made the success of this conference possible.
Corresponding author Weibing Ding, PhD, is a director at GlaxoSmithKline, [email protected]. Martina Micheletti, PhD, is an associate professor at University College London, [email protected].
You May Also Like





