Choosing Between Single-Use and Multiuse Technologies: An Interphex 2015 Roundtable Discussion
December 18, 2015
On Tuesday, 21 April 2015, Eric S. Langer (managing partner at BioPlan Associates) chaired a midday roundtable titled, “Deciding on Single-Use vs. Stainless Steel Bioprocessing Strategy: What Do CMOs Know That Biopharmas Don’t?” Langer brought together several industry experts to discuss choosing between stainless steel and single-use technologies (SUTs) for different applications:
William Hartzel (director of strategic execution at Catalent Pharma Solutions)
Steven Perry (vice president of technical operations at Cook Pharmica)
Joanna Pezzini (bioprocess engineer at MedImmune)
Daniel Vellom (senior director of global technology innovation at Sanofi Pasteur)
Sue Behrens (senior director of process technology at IPS-Integrated Project Services, Inc.).
Eric Langer (BioPlan Associates)
The decision process regarding whether, when, and where to use stainless steel (SS) and single-use systems (SUSs) for biopharmaceutical manufacturing is complex. So Langer began by introducing the panel’s “true biopharma experts who have made these decisions.” He prefaced the roundtable with a single slide listing “what contract manufacturing organizations (CMOs) know” about this decision process. No convincing data yet suggest that one technology always outperforms others for product safety, cost-effectiveness, or operational efficiency. And most biopharmaceutical development companies don’t have their own in-use data to consult in making strategic manufacturing decisions. But “CMOs are paid to be efficient, knowledgeable, cutting-edge, and flexible,” Langer said. Their SUS–SS decisions often require carefully defining platforms for their manufacturing strategies.
Finally, he showed a chart from his company’s most recent annual survey, wherein companies were asked what types of bioreactors they were most likely to consider for clinical and commercial scales in upcoming new facility construction. For fed-batch culture, over 70% of respondents chose single-use technology for clinical scale, and nearly half chose it for commercial scale. Compare that with just over 30% choosing stainless steel at clinical scale and about 58% choosing it for commercial scale. With interest increasing in perfusion culture, over a third of respondents preferred single-use approaches to that concept for clinical scale, whereas just under 20% preferred stainless steel. For commercial scale, the number of answers were about tied for both types of perfusion technology.
William Hartzel (Catalent Pharma Solutions)
Hartzel advised focusing in the beginning on your goals and objectives in a construction or manufacturing process — “what you need to deliver.” They are typically different for innovator companies and service providers. “When we looked at going from stainless steel to single use,” he said, “we considered the fit and philosophy around our business models, then how we could align our business and our manufacturing strategies together. One of our main focuses is providing flexibility to our customers in a multiproduct facility. We have a cell expression technology based on getting products to clinic fast.”
He provided some history to describe Catalent’s “unique situation” in deciding to implement SUSs. “Our site has gone through three major expansions since the early 1990s. Catalent began as an organization focused on transgenic technology and went from manufacturing in cattle to SS reactors. So for us to jump from SS reactors to single use wasn’t as large a jump as we had already made.” The company was ready to expand in 2008, but then the market crashed, putting a hold on that project. “We had already ordered our long–lead-time items.” Catalent bought six SS reactors before putting the project on hold. By 2011, the market opened up again, so the company was ready to grow.
This expansion plan covered the phase 1–2 stage of manufacturing, and the company wanted to incorporate additional cell expression technologies. In its design philosophy, “Not only was it about getting bigger, but it was also about getting smaller.” Hartzel said the company was looking for turn-around efficiency. SUSs provide energy and labor savings with a corresponding increase in consumables (Table 1). But Hartzel said that his company prioritized flexibility.
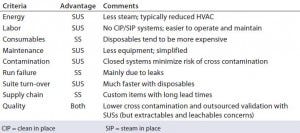
Table 1: Advantages and disadvantages of single-use systems (SUSs) and stainless steel (SS) according to Hartzel B. Embracing Single Use As a Strategic and Manufacturing Philosophy. Interphex 2015 (New York, NY, April 2015) — “Generally speaking, single-use facilities are more flexible than traditional facilities, a major advantage in a multiproduct facility.”
In 2011, Catalent engaged an engineering firm to analyze its options. The findings: Despite previous investment in SS reactors, the capital and time required for their supporting infrastructure still made the SU–SS decision neutral regarding capital expenditures. Choosing SUSs reduced some risks in facility construction and better aligned the company’s manufacturing strategy and business plan. Catalent derisked the build through simplicity and a compressed timeline and lessened client concerns over cross contamination. And it would provide labor savings for simplified suite change-over (especially through the elimination of clean-in-place procedures) as well as reconfiguration options.

Photo 1 and 2: Catalent expansion, June 2012, Catalent expansion, June 2013
The moment of truth came when the company sold its SS reactors to a competitor. The expansion program accelerated at that point, going from “a slab of concrete and the inside structure of a building” to a good manufacturing practice (GMP)-compliant, revenue-producing facility in just one year (Photos 1 and 2) at “a fraction of the cost of what a SS or fixed system would have ended up costing.” Closed systems have improved the efficiency of this multiproduct facility. “We can handle nine different products at one time,” Hartzel said.
The company learned several lessons through this expansion project. Hartzel emphasized working closely with equipment suppliers as partners. “We were able to get a loan from our (bioreactor) submanufacturer.” Ultimately, he said, SUS buyers are outsourcing elements of validation. “You’re not buying bags or tubes; you’re buying validation packages.” This requires a high level of confidence in the supplier.
Supply chain matters are critical, he said, from change control and inventory management to employee training in how to handle SUSs. Communication was key — both internally and externally (with suppliers and customers).
One audience member asked about the “validation package” concept. Hartzel pointed to change control and supplier auditing. SUS buyers need to know that their vendors have the right level of controls in place. That gives a buyer confidence in getting the same results over time.
The moderator asked, “What single point would you share with a biopharmaceutical company transitioning from SS to SUSs or deciding between them? What one point would you recommend that they consider?” In answering, Hartzel went back to his initial point about focusing on end goals. For example, running an individual process consistently over time is different from a multiproduct approach. “I think that flexibility and elimination of cross-contamination potential are the main drivers,” he said, in choosing SUSs.
Steven Perry (Cook Pharmica)

Photo 3: Cook Pharmica drug-substance upstream (production) suite
Perry began by describing his company and its history. Established in 2004 as a division of Cook Medical, Cook Pharmica began as a contract manufacturer of drug substances and has since added development laboratories and drug-product formulation, fill, and finish capabilities — all in one location. The company has nearly a million square feet of facility space in two large buildings. The campus used to be the largest television assembly plant in the world. “It was shut down and gutted,” Perry explained, “and we turned it into a state-of-the art biomanufacturing facility.” This leaves room for expansion from the current 2,500-L bioreactors to larger scales.

Photo 4: Cook Pharmica drug-stubstance downstream (processing) suite
The company operates a hybrid facility using both disposable and reusable equipment and components. Buffer and media preparation and postfiltration storage heavily involve single-use technology. “We prepare all our buffers and media ahead of time, then sterile filter,” Perry said, “and they are then used as needed by our operations.” The seed train is mostly single-use as well, up to the 200-L scale, with both rocking and stirredtank disposable bioreactors. In-process mixing for downstream processing is also SUS-based: e.g., for low-pH and acid viral inactivation steps and productivity adjustments. All mixing and in-process storage are based on single-use technology (up to 1,000 L), as are almost all filtration operations.

Photo 5: Cook Pharmica drug-product manufacturing (fill-finish) suite
Stainless steel and reusable technologies do have their place at Cook Pharmica. The 2,500-L production reactors and 600-L “N – 1” reactors are stainless steel. Disposable bioreactors were still somewhat unfamiliar when the company started 10 years ago. Centrifugation and harvest tanks are also reusable, as are chromatography systems. Ultrafiltration/diafiltration (UF/DF) operations are the only filtration operations using stainless steel rather than single-use technology. All this equipment is traditionally cleaned and sanitized between uses.
The most commonly cited rationale for implementing SUSs is elimination of cleaning validation/verification for rapid change-over between manufacturing runs. Perry also pointed to the flexibility that can help a contract manufacturer accommodate multiple processes. Then he highlighted scheduled decoupling of processes with the example of media and buffer preparation.
A CMO must accommodate a number of different types of processes. Some use many different buffers and media; others are fairly straightforward, with fewer buffers and media involved. Single-use technology allowed this company to decouple preparation operations from actual processing, leveling out labor needs by scheduling those operations well in advance of when the materials are actually needed.
Using the same example, Perry also talked about risk mitigation. With media or buffers sterile-filtered into presterilized bags for holding, they are stored under appropriate conditions rather than going straight into a bioreactor or process stream. Decoupling preparation and use lessens the risk of process contamination. The company can perform bioburden testing, for example, before using a buffer or medium. If a problem arises, it would not affect an actual bioprocess.
Perry then went on to discuss challenges associated with single-use technology such as those relating to inventory management and supply chain. “You are almost certainly going to be dealing with some type of assembly or bag that has been customized in some way,” he said. So working closely with vendors becomes critical. But a CMO faces lead-time issues if it’s working with many different configurations and custom assemblies. Making sure to have needed components on hand can increase inventory costs. “Managing supply chain and inventory has been very important for us.”
Supplier quality is important as well. SUS users rely heavily on their vendors’ expertise and quality systems. Users are outsourcing part of the assembly process, Perry said, as well as the validation Hartzel mentioned. “It is important to consider your supplier as a partner. We audit vendors and make sure that they have the right quality systems and testing in place.”
Design limitations also complicate matters. Perry offered the example of single-use bags with integrated tubing. “What is your flow out of that bag?” Often that can be limited by inlet and outlet tubing size. If that doesn’t match the bag volume, flow can collapse inlet tubing or you can over-pressurize outlet tubing.
Set-up presents some difficulties, and operators need special training and plenty of practice in handling single-use technology. Returning to the bag example, Perry explained that incorrect set-up in single-use mixers can cause bags to wrinkle or fold and tubing to kink or twist. “Operator training,” he said, “goes hand-in-hand with a number of new aseptic connectors” and disconnectors, some of which require specific techniques in use. “You are relying on your operator to make an aseptic connection correctly,” Perry cautioned, “and you could be introducing some risk into a process if operators are not trained or don’t have enough practice.
Finally, he pointed to potential interactions between plastic materials and bioprocess fluids in contact with them. “That can particularly be an issue sometimes in upstream processes, where there may be some impact on cell growth. We’ve had to work around that on occasion.” Perry highlighted the importance of small-scale and process development work before implementing SUSs at large scale.
Cook’s general philosophy, he said, is to use disposables where they are well established and low risk while conferring a true operational advantage. “We are continually evaluating single-use applications,” he said, citing a potential move to larger disposable bioreactors. “But that’s going to take a lot of work and evaluation.” And he said that companies should not overlook operational details. Practice trials, engineering tests, and operator training are highly important in implementing SUSs.
An audience member asked for more detail on Cook’s decision-making process and the hybrid facility that resulted. “As a CMO, we have to have flexibility,” Perry answered, “but it’s also important that we have reliability for our customers.” If a batch fails, clients don’t care whether single-use or traditional equipment was involved. So Cook uses disposables where they provide flexibility but remains somewhat risk averse. “You’re only as good as your last customer,” Perry quipped. He said his company has chosen to stay with stainless steel wherever few single-use offerings are available: in centrifugation and chromatography applications, especially. “We are not ready to make the leap for those.”
When asked to offer “one piece of advice” for companies facing these same decisions, Perry recommended that they be very clear on what they are trying to accomplish. “Why are you implementing disposable systems?” he asked in turn. “Is it just because that seems to be the way the industry is going? It is important to look at new technology, but the rationale has to be from the operational side.” SUSs should provide flexibility or solve particular facility concerns. “But don’t underestimate the implementing challenges,” he warned.
Joanna Pezzini (MedImmune)
Pezzini’s single-use experience is primarily in downstream processing. Her group performs related technology transfer activities. “We have been looking at a single-use UF/DF skid for a 500-L GMP facility,” she said, “for a 2,000-L GMP facility, and then also for a 500-L pilot facility.” The GMP facilities are primarily based on stainless steel equipment (with the company looking to add disposables), whereas the pilot facility is more single-use overall. “We have some single-use bioreactors and some stainless steel,” Pezzini said. “We primarily use bags for buffers and a lot of tubing connections.”
She reviewed some drivers behind decision-making related to the single-use UF/DF skids, highlighting costs and benefits as well as associated time savings and technological challenges. The primary driver for purchasing a single-use UF/DF skid actually was its increased operating range over that of older technologies. Pezzini pointed to its smaller hold-up volume relative to that of the stainless steel units her company considered. “This allows us to achieve much higher concentrations or work with much lower production titers.” The lower-cost and portable single-use skid works with multiple bag and tubing sizes, providing a broader range of volumes and pump flow rates. It fits through a 40-inch door, making it good for MedImmune’s smaller suites and doorways, and it weighs less than the stainless steel option. “We found that we could move it between GMP and non-GMP facilities,” Pezzini said.
Capital costs for the single-use skids are about 50–66% of those for a stainless steel system. “But then,” Pezzini cautioned, “you have consumable costs. Every time you want to run a batch, you need to get a new tubing kit with all the instrumentation, pump heads, and so on.” She said MedImmune expects to break even running about 30 batches on the new system. How cost-effective that is depends on how many batches a company runs each year.
Pezzini said her company is sticking with reusable filter membranes, however. A cost analysis comparing single-use and reusable options showed that the former were more economical only for single batches. If disposable membranes are cleaned and reused like traditional membranes, she explained, then the cost savings is lost because they are slightly more expensive and that difference is made up only if cleaning and cleaning validation costs are eliminated. “We also find that as we move up in scales,” Pezzini said, “we get slightly lower break-even points: fewer batches before single-use and stainless steel systems become equivalent in cost.”
Single-use technology provided the company with substantial savings by eliminating the cost of cleaning validation. That also saved time in commissioning in the GMP suite. “Because we can move this skid from the pilot facility,” she said, “we did all of our commissioning and testing and automation work there.”
Single-use tangential-flow filtration (TFF) does take more time to set up than a stainless steel system because the latter has filters already installed. Stored in hydroxide, they simply need cleaning. “Because we are using reusable membranes,” Pezzini pointed out, “we still have to clean our system beforehand. We haven’t found a suitable single-use membrane. But when we find one and manage to validate that in our process, then we expect the set-up time to reduce by about half.” It would then take about two hours for two people to set it up and operate it.
The team found operation of the SUS to be easy, with smooth automation. “It was basically just set it up and let it run.” Pezzini described this as quite different especially in the 500-L GMP facility, where the previous system was manually set up and run. “Automation was one of the drivers to purchasing a new skid, and we really did achieve that.”
Challenges: “For us it was new to need an extractables and leachables assessment,” Pezzini explained. “That took extra time. We also had to have a new instrument calibration approach.” Disposables users rely primarily on vendor calibrations and certificates for the UF/DF instruments. “We also needed a new post-use calibration approach,” she said. “We don’t do it on all the instruments, but we are still doing it for the critical ones (e.g., pressure indicators). We’ll have to see how that moves forward based on the data we collect over time.”
Pezzini said that another concern that came up is kit reliability. “It hasn’t been perfect,” she admitted, “but it’s been pretty good.” And her team has seen lower pressure ratings for the SUS. Thus, operating at a pressure limit requires slightly lower flow rates than a stainless steel system could accommodate. “This isn’t across the board all the time,” Pezzini said, “but in a few instances it might come up and make the process step a little bit slower.”
Accuracy of single-use turbine flow meters is another issue. MedImmune has used flow meters to calculate diafiltration volumes, but her team has found these not to be accurate enough for that purpose. “We’ve had to use to a floor scale to collect that volume and accurately measure it. We are not currently aware of a disposable flow meter that could be a replacement for this.”
Finally, Pezzini highlighted a problem with mixing in single-use retentate bags. It seems to be efficient only up to a 20-L bag volume because the system relies on retentate flow alone for mixing. That is inadequate for high-concentration solutions, with their low flow rates and high viscosities. “We are looking at different mixing systems to use with the system at the 2,000-L scale. We are covered at the 500-L scale, where the system is already implemented. But for the 2,000-L scale, we are looking for active mixing with an impeller in a bag. We are considering specific impellers because we have found some shear issues with certain designs.”
The single-use skid provides greater flexibility, Pezzini said. “We could get one skid instead of two at our 2,000-L scale because it can be moved around very easily.” However, she cautioned, cost savings depend on how much use a skid gets. “We still do have some technology challenges, but we feel that we can get through all of them. We will get these systems working well.”
The moderator asked whether Pezzini, with personal experience working for both a CMO and a biopharmaceutical development company, could address the differences between how one or the other might consider these technologies. She said that the difference may not be as great as expected. “Different budgets are larger or smaller than others, so the capital savings can be either more or less important. It depends on budget and how the economy is doing and so on.” An established company with a great cleaning system in place may not see great benefit in going disposable. But for a new facility — or whenever flexibility is important (e.g., in pilot facilities) — disposable equipment becomes much more attractive. Finally, Pezzini brought up concerns regarding supply chain and lead times. “We are bit concerned that, if we need to get parts quickly, it might not happen.”
Daniel Vellom (Sanofi Pasteur)
Vellom offered the perspective of a vaccine company considering single-use and stainless steel technologies for the many different products it makes. He called his industry conservative when it comes to technology. “Most of our products go into healthy people and babies, so we have to be extra cautious.” The vaccine industry also deals with several different types of products: e.g., killed or live-attenuated pathogens; virus-like particles; protein and polysaccharide antigens; and even DNA and RNA vaccines. So decision-making is highly product specific.

Photo 6: Sanofi Pasteur mobile suite
Vellom said that the main advantage of single-use technology is related to initial capital expenditures when building or expanding a facility. It eliminates a great deal of stainless steel piping (e.g., clean steam and clean-in-place systems). “You are building a clean room,” he said, “almost stripped bare, with some utilities (gases, air flow). But you don’t need all that stainless infrastructure and piping. And you don’t have to validate and clean it and continuously revalidate.” In vaccine facilities, no two suites are the same because each one is custom designed for a given product, making disposables an attractive idea. Vellom highlighted the concept of modular units in SU facility design, pointing to G-Con Manufacturing’s mobile suites as an example (Photo 6) that can be moved around, reconfigured, and connected.
But he cautioned that when the product-contact surface comes from a vendor, that shifts the responsibility to the vendor to provide exactly what is needed. And disposables bring with them new concerns over leachables, extractables, and particulates.
Some vaccines are more amenable to SUSs than others: e.g., viral vaccines produced in cultured (often insect) cells and some bacterial toxins/antigens. “We can culture cells and propagate viruses in single-use bioreactors,” Vellom said, “but there aren’t single-use centrifuges or column chromatography systems that work at the scale and speed we need. So we always end up with a hybrid system: some stainless or CIP/SIP-capable equipment comingled with single use.” Mass-transfer rates for anaerobic bacteria are similar to those of mammalian cells, but the related demands of anaerobic bacteria are too high for single-use bioreactors.

Photo 7: Single-use bag and tubing in use at Sanofi Pasteur
SU operations can be a challenge. “A different level of training is required of operators,” Vellom said. “Rather than a large piece of stainless steel in a steam plant, you’re now working with complicated, relatively fragile bags and tubing and manifolds. You can end up with a lot of ‘spaghetti’ on the floor (Photo 7). It’s easy to tear or stress a bag getting it out of its package and put into place.” Operators must learn to be careful with disposable components. He said his company has helped vendors improve their packaging with this in mind.

Photo 8: Single-use bioreactor set-up at Sanofi Pasteur
“We’ve had to work with vendors and operators,” Vellom said, “putting them in the same room to figure out what they need from each other.” He described a bioreactor example, with which some components seemed to vary in robustness over time. “We’d have two or three runs that went well, and then all of a sudden in the next run something would crash, and we’d get a contamination. It turned out that some of our processes were stretching the limit for what had been tested, so we tore things up not really recognizing where the weak points were.” Storage space also can be a problem. Because packaging must be robust, component inventories can take up a lot of space.
When asked to provide one piece of advice in considering disposables and stainless steel options, Vellom said that he would encourage companies to involve their quality and materials management groups from the beginning. “Bring them into the room, get the conversation started early. It’s no fun to get six months or a year into a development project and then have to go back and explain to quality or materials management groups why you chose this or did that.” All perspectives feed into change control and flexible manufacturing.
An audience member asked about appropriate training programs. Vellom said Sanofi brings suppliers in to train its operators. “They know their products, they know how to deal with them because they designed them.” Lunch-and-learn programs every other month or so bring in a vendor when the company scales up or begins working with something new. “Use the expertise that’s out there,” he advised, “and run programs that put operators in contact with the people who made the products. Don’t try to be an intermediary; you’ll end up chasing your tail.”
Another attendee asked about increased manual operations with SUSs relative to stainless steel. Vellom pointed again to training. “A stainless steel bioreactor skid is a fixed piece of equipment, and all you are doing is changing what you’re flowing through it at any given time. It’s all set up with valves and automated systems: If I want CIP, then I push the CIP button.” But single-use technologies involve complicated tubing manifolds and so on. Especially for aseptic operations, those must come out of the packaging and maintain integrity throughout set-up and installation. That is a highly manual and technically demanding operation, as is breaking it all down and removing things after a process is complete. Operators need to be nimble, and standard operating procedures (SOPs) need to be specific.
Another questioner brought up bioburden. Vellom said his company has no tolerance for it. “If I have any bioburden, then I start over.” When viruses are the product, bioburden cannot be filtered out, which makes closed-system and aseptic processing necessary.
Next, someone asked about failure rates. “We get better with time and training,” Vellom asserted. One commenter said that companies relying on stainless steel typically achieve 95% success rates. And Vellom confirmed, “We get there, too.” With 50-L to 1,000-L single-use bioreactors, he said, training can bring a company to similar success rates. “There might be a slower start or learning curve. At the beginning there is a lot more to worry about. But ultimately we get there, and we can run consistency batches and improve the product.”
When asked about supply chain issues, Vellom admitted that users of disposables depend on their suppliers to deliver needed components when they are needed. “We need it to come in, and we need it to come on time.” His company spends time on supplier management and keeps a certain number of components in inventory. Gamma-irradiated components typically have a two-year shelf life. And, Vellom pointed out, “we are really friendly with our key suppliers.”
Sue BehrenS (IPS)
Behrens described a client’s project for the US Department of Defense involving advanced development in manufacturing for medical countermeasures. The company built a facility in Florida to “make anything, any time, in any amount — and we don’t know what it is yet.” The initial question was how to design a building that could accommodate any platform process and respond in under three months to make a first dose. Behrens said that such a project was well-suited for single-use technology.
Many people make assumptions about SUSs, and those blanket statements are true sometimes. Behrens addressed in detail some “bold assertions” people make when comparing single-use and stainless steel equipment: speed (disposables are faster to implement), cost (stainless steel is more expensive), open architecture (single-use is more amenable), flexibility (stainless steel facilities are less nimble), and equivalency (operating results are comparable for both).
Which Is Faster? Behrens elaborated on the need for speed. “We have strategies and needs for faster filings.” During 2014’s Ebola scare, some developers were getting product ready to go in three to six months. “We have that with flu,” Behrens said, “and we need that for pandemics, for anthrax, and other response items.” Many companies are looking to disposables to help speed things up. But it isn’t that straightforward.
“Single-use isn’t always the faster answer,” Behrens said. Extractables and leachables studies can be extensive, for one thing. “If you already have a stainless steel system up and running, then you’ve got your CIP and SIP systems in place. You’ve already spent the money, and it’s actually pretty quick to get a process into stainless steel, as well.” Management must think hard about previous investments, about the stage of a given manufacturing process, and about their own company’s specific business case.
Which Is More Economical? Certain costs are reduced with single-use technology. Capital investment is initially lower. The same process can be developed in a smaller facility, which can be expanded later when the probability of regulatory success is improved. Companies should be able to match their spending to the demands they need to meet when they need to meet them.
Behrens spoke of the risks associated with building dedicated single-product facilities. “I spent 20 years at Merck,” she recalled, “as we built three huge buildings to make a herpes zoster vaccine (Zostavax), the Gardasil recombinant human papillomavirus vaccine, and a rotavirus vaccine (RotaTeq). We started building 10 years before those products were licensed. We spent billions of dollars to get ready for those product launches.” Single-use technology would have provided an opportunity to push some of that investment forward, spending the money when the product is closer to licensing without risking a supply shortage when it is approved for market.
However, single-use operating expenses can be higher, particularly when it comes to consumables costs, waste disposal, labor associated with manual handling, storage space, and inventory management. The facility-size advantage decreases with scale, and companies must also consider the cost and availability of equipment and components over time. Behrens mentioned that for many batches, it may cost more to buy big, complicated, and expensive bags than it would have cost to buy reusable stainless steel equipment. “And you have to make that decision before you know,” she pointed out. “So how do you make that call? You have to sit down with your marketing and regulatory people and try to understand where your product is going. It is highly driven by your business decisions.”
What About Open Architecture? In so-called “ballroom facilities,” all bioprocess operations would be performed in one large space using closed (single-use) systems. Imagine operators who are cross-trained to transition between upstream and downstream operations, walking from a chromatography skid to a bioreactor and then over to a TFF skid.
“That starts to get challenging from an operational point of view,” Behrens cautioned. “These are all complex unit operations.” Each requires a lot of training on its own. She pointed to earlier speakers’ points about the importance of training. If something goes wrong in a ballroom facility — e.g., a leak or power outage or skewed environmental monitoring — that could affect the entire process rather than just one unit operation.
No matter how big or small a facility, upstream and downstream have always been different specialties. Lean-manufacturing strategies may suggest that cross-training is possible, but specialized expertise cannot be underestimated or undervalued. “If it’s really just a quick valve-switch,” Behrens said, “you can probably count on someone to run over and switch the valve without a fundamental understanding of the unit operation.” But she questions whether companies want to lose the value of building long-term expertise in different areas.
“We still don’t have full SUS acceptance in all large companies,” Behrens said. Regulatory agencies claim to accept single-use technology and the ballroom concept with proof of closed processing. But the industry needs education to alleviate concerns over associated business risks. “Some biological products are worth millions of dollars,” Behrens explained. “When you think about the revenue lost when you have a problem and have to shut down everything, you are really putting your business at risk.”
Which Is More Flexible? SUSs are said to offer flexibility in operations. Referring back to the DoD project, Behrens said, “We believe that our facility in Florida does have a lot of flexibility. For example, we have upstream and downstream processing modules. Today you can put something in downstream, tomorrow in upstream, then both if you have a small process. You can do them all in a ballroom. You certainly can’t do that with stainless steel.”
She also mentioned global supply chain distribution, citing vaccines as an example. Many such products are not shelf stable, and some do not do handle shipping well. But the people who need vaccines are not clustered around the few plants that make them. It would be helpful to reproduce biomanufacturing processes in facilities around the world. “When it comes to a pandemic,” Behrens pointed out, “the first response is to close all borders. So if you don’t have manufacturing for a vaccine inside a given country, you’re out of luck because nobody will be moving anything around.” SUSs could make it possible to reproduce processes at widely distributed small-scale facilities.
However, some caveats come with that idea. A process is constrained to what’s available from technological innovators. For example, if a process requires a certain ion-exchange resin that is unavailable in disposable format, then that process cannot be entirely single use. “You might be able to get somebody to prepack a column for you,” Behrens suggested, but it takes time to develop and put together a fully validatable package for something like that.
“We talk a lot about small scale,” she went on, “and there is definitely a break in scale for applying this concept.” Biosimilars are likely to be large-scale products through the foreseeable future. Some innovative biopharmaceuticals will continue to be large scale as well. But many vaccines could be amenable to small-scale bioreactors.
Behrens pointed to classic roller-bottle cultures as a type of single-use technology. “We’ve been using them for hundreds of years,” she said. Referring back to questions about success and contamination rates, she reminded the audience that “you open every single bottle, thousands of them per lot, at least six times in a manufacturing environment. So we know how to deal with disposable reactors.” For vaccines, that knowledge can be applied according to scale and product and business model.
Are Results Truly Equivalent? “We hear from our clients all the time,” Behrens reported. “They say, ‘We don’t need to worry about it; we’re going to use single-use.’” The consultants’ response is to ask how a client thinks it will accomplish that. How will product be transferred from upstream to downstream process, for example, and how will samples be taken? And the response is often to shrug those questions off: “Oh, we’ll address that later. We’ll figure it out.”
But those considerations all take time and thought to work out. Even if a company can get the same end product of the same quality, taking a different journey to get there requires specialized training, quality systems, and batch records. “You need to think about the tubing as it comes across and lies on the floor,” Behrens said. “Is that a quality problem? I think your quality organization will tell you that it is. So you need to figure out how accommodate those tubes. How are you going to manage all those connections and make sure that the right ones are made?” Radiofrequency identification (RFID) tags may offer a solution to some questions. “That’s the kind of tool we are looking for to facilitate operations.”
In regard to the question of single-use technology, Behrens concluded that it’s not the solution for everything, every time, and everybody.
Panel Discussion
A brief panel discussion followed the presentations, in which one audience member asked whether the panelists were generally pleased with the process performance of single-use bags. Perry spoke primarily of smaller-scale operations. “We normally use single-use bioreactors in the seed train. And we did have, at least in one case, an issue with leachables and compatibility. It’s a very sensitive part of the process. You can’t affect cell growth. So we had to take some measures and pretreat that reactor for a phase 1 process. You certainly wouldn’t want to have to do that for a large-scale commercial process. So if the problem were to go forward, we would have to go back and work on it. Sometimes you need those kinds of work-arounds.”
Failure Modes: When a stainless steel reactor is contaminated, it’s usually caused by a component that fails or didn’t get fully sanitized. But failure modes are different with single-use technologies. “You can be pretty sure that the bag you get has been presterilized,” Perry said, “and we haven’t had an issue with gamma-irradiation failure.” Where issues do occur is in the interconnections between bags and sample lines or outlets, for example. Steam connections are problematic: If operators don’t account for temperature differences, connections can loosen over time. “Overall,” he concluded, “I think we have good performance. But you have to watch out for some things.”
Vellom spoke up too, saying that the question revolves around robustness as well as performance. The latter is measured in terms of mass transfer and a general ability of a single-use bioreactor to support cells (for example) in the same way that a stainless steel reactor would. “But there is also a question of robustness,” Vellom said. “I recently had an incredible experience. Our partners at Genzyme are working on extended (semicontinuous) processes. They ran a 50-L single-use bioreactor for 160 days straight, stopping only because of the holidays. And it just kept going. That gave us confidence in the robustness of some of these components, how they are put together and engineered.”
“What cell density were they working with?” asked one attendee. Vellom said the process has used Chinese hamster ovary (CHO) and human embryonic kidney (HEK) 293 cells in the range of 20–30 million cells/mL. “How many manipulations were performed in that 160 days?” the attendee wanted to know. And Vellom described the process as a “fancy perfusion” based on continuous feed of fresh media. A custom-designed sample port periodically draws samples so operators don’t have to repeatedly penetrate the bag. The sampler is fixed in place and automated. Genzyme uses spectroscopic methods to monitor metabolites and viable cell density. “They spent a lot of time engineering out those risky manipulations as much as they could,” he said.
Making Connections: Another audience member asked about continuous downstream processing, particularly focusing on hardware connectivity. Vellom described a hurried post-9/11 development project for a smallpox vaccine. “We spent a lot of time sorting out the connectivity issue,” he said, “assessing technology availability and training. We had tubing welders everywhere, trying to figure out which was the most robust. We spent a lot of time building little manifolds with a valve on the end and a piece of tubing that we could weld on, autoclaving and steaming it on. We managed to do it, but that’s not the way you’d want to start today.”
Since then, vendors have come out with aseptic connectors, disconnectors, and so on, so biomanufacturers don’t have to rely on tubing welders. “The more you move to those kinds of built-in solutions,” Vellom pointed out, “the better off you are. Your choices are a lot better now.”
Cheryl Scott is cofounder and senior technical editor of BioProcess International, PO Box 70, Dexter, OR 97431; 1-646-957-8879; [email protected]; www.bioprocessintl.com. Photos used herein are property of the respective companies: Catalent, Cook Pharmica, and Sanofi Pasteur.
You May Also Like





