Certified Reference Mixtures in Extractables Screening of Polymeric Materials: For Container–Closure Systems and Single-Use Equipment
 Plastics have been used for decades in container–closure systems (CCS) for drugs and in single-use (SU) manufacturing equipment for biopharmaceutical processing. Biomanufacturers must comply with national and international laws and regulations that require proof that use of such polymeric materials is safe (1, 2). That necessitates testing for potential biological responses and interactions with drug substances. Comprehensive extractables and leachables (E&L) studies also are required for potential release of undesired compounds from polymers.
Plastics have been used for decades in container–closure systems (CCS) for drugs and in single-use (SU) manufacturing equipment for biopharmaceutical processing. Biomanufacturers must comply with national and international laws and regulations that require proof that use of such polymeric materials is safe (1, 2). That necessitates testing for potential biological responses and interactions with drug substances. Comprehensive extractables and leachables (E&L) studies also are required for potential release of undesired compounds from polymers.
Extractables tests are performed on materials under laboratory conditions that accelerate or exaggerate normal use conditions (3). These tests provide a comprehensive overview about compounds (extractables) that could be released into a liquid (e.g., drug product) that comes into contact with the material. Results are used for materials risk assessment and are the basis for the design of a targeted leachables study.
The aim of a leachables study is to detect and quantify compounds released from plastics that are present in a drug product. Such studies are conducted in a good manufacturing practice (GMP) environment on multiple batches of a drug product under its use conditions (e.g., formulation, storage time, and temperature).
The challenge in extractables testing is the multiple, structurally diverse compounds that ideally must be screened for in one chromatographic run. Further, sample matrices vary, and different extraction solvents are used. The analytical toolbox routinely applied includes gas chromatography–mass spectrometry (GC-MS) and liquid-chromatography–mass spectrometry (LC-MS). The screening method requires that researchers define a range of compounds to be analyzed. The method then can be validated by selecting representative analytes of each expected compound class. For polymer analysis, the selection should include typical and relevant extractables (e.g., if they are of toxicological concern or ubiquitously present). A representative of each relevant chemical class of extractables should be included in the study.
With such a mixture of compounds, researchers can determine the most relevant performance parameters, including linearity, repeatability, intermediate precision, limit of quantitation (LoQ), and limit of detection (LoD) for individual compounds (4). But more important, researchers can estimate the “fit for purpose” of an analytical method for a respective chemical class of individual extractables. For example, linear alkane dodecane can be used as representative of the chemical class hydrocarbons, and octanoic acid can be used to estimate the method performance for aliphatic acids.
To obtain reliable and accurate results in such measurements, reference methods are used, and certified reference materials (CRMs) should be applied whenever possible. Because CRMs have not been established so far for plastic materials and typical plastic additives, MilliporeSigma developed CRMs encompassing most common and relevant E&Ls as two individual standard mixtures adjusted for use on GC-MS and LC-MS instruments.
Below, we highlight the development and certification approach for CRMs and how they can be used in method validation and routine quality control (QC). We provide technical details for the instrument and method setup to detect CRMs used in LC-MS analysis because liquid chromatography–high resolution mass spectrometry (LC-HRMS) is the most difficult analytical technique applied in E&L testing.
Composition of E&L CRM Mixes
Specifying the composition of a reference mixture that is fit for purpose for most laboratories involved in E&L testing is a complicated task. Over the past few years, a tremendous pool of knowledge for commonly used polymeric materials and expected extractables has been generated (5, 6) or has become available as material information (7). That provides a consistent overview of chemical classes that need to be covered in a polymer investigation.
Compounds for the E&L screening standard were carefully selected to represent a large variety of polymers, plastic additives, and processing aids. Further, it was ensured that such compounds can be detected with at least one common detector used for both LC and GC techniques. That is relevant, for example, to LC-ultraviolet (UV)-MS systems to be able to detect compounds by means of their UV signals if they cannot be ionized and detected by MS. Table 1 shows the compositions for the E&L screening standard for LC and GC, including classification to the chemical class and common uses and origins. The selection includes typical antioxidants and degradants, processing aids such as releasing agents, curing agents, plasticizers, UV absorbers, and polymer monomers. Most chemical classes that can be encountered in E&L studies are covered by the compound selection.
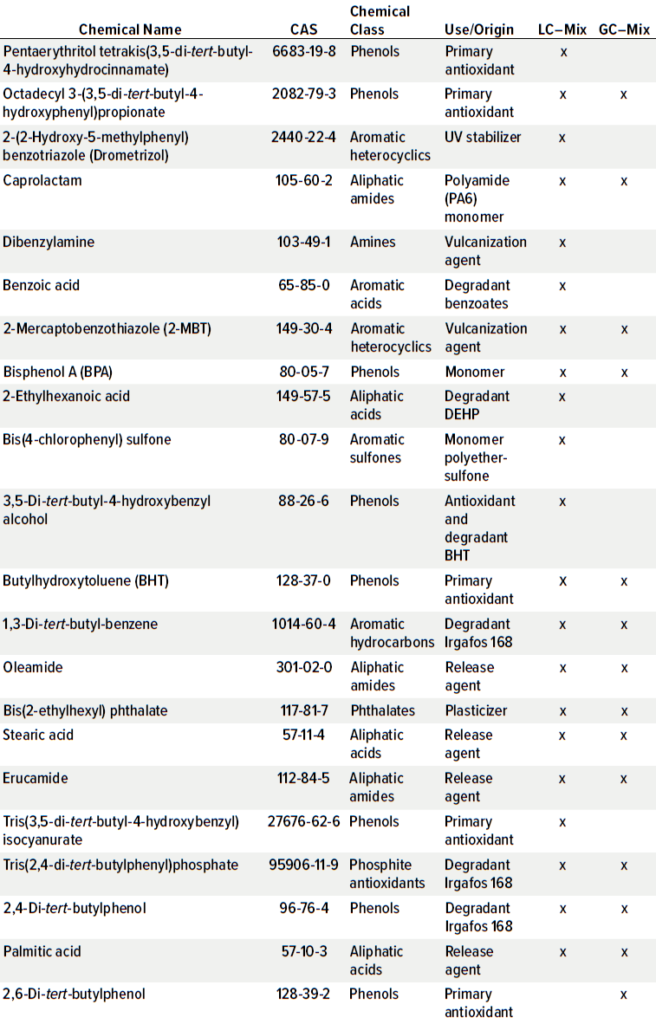
Table 1: Composition of extractables and leachables (E&L) screening standard for liquid chromatography (LC) [95636] 21 compounds (50 μg/mL per compound) in acetonitrile and E&L Screening Standard for gas chromatography (GC) [01829] 14 compounds (50 μg/mL per compund) in tert-butylmethylether (Irgafos is a trademark of BASF).
Some typical plastic additives listed in US Pharmacopeia (USP) <661.1> and European Pharmacopoeia (EP) 3.1.13 Plastic Additives are available as pharmaceutical reference standards. A reference mixture with few compounds according to ASTM standard D6042-96, which is dedicated for testing of polypropylene, also is available. Such individual reference compounds or the ASTM mix are designed and useful for material characterization toward known additives — thus, target methods but not necessarily for screening purposes.
Manufacturing Process for CRM Solution Mixtures
Customers require standards or reference materials that are well characterized to perform accurate analytical measurements. CRMs are characterized with the highest precision and can be traced back to International System of Units (SI units) by means of primary calibrators issued by national metrology institutes (NMIs). Figure 1 shows the different quality levels from research chemicals to CRMs and primary materials, which together provide the highest level of accuracy and traceability.
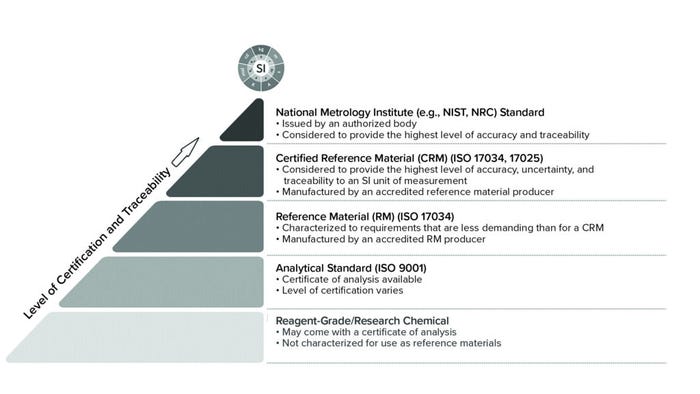
Figure 1: Documentation and traceability of the different quality levels of chemicals (NIST = National Institute of Standards and Technology, NRC = National Research Council, ISO = International Organization for Standardization).
The development and preparation of CRMs must follow manufacturing processes under International Organization for Standardization (ISO) 17034, and all analytical tests within that process must be carried out using ISO/International Electrotechnical Commission (IEC)-17025–accredited analytical methods (8, 9). As Figure 2 shows, the workflow starts with the synthesis and purification of different components in the mixtures and ends with the generation of a certificate.

Figure 2: Complexity and workflow for the generation of a certified reference material (CRM) (LC = liquid chromatography, GC = gas chromatography, MS = mass spectrometry, IDMS = isotope dilution mass spectrometry, qNMR = quantitative nuclear magnetic resonance, wb = within bottle, bb = between bottle, AST = accelerated stability testing, LTS = long-term stability).
Primary absolute or primary ratio methods are required to determine the content of the compounds in the CRM mixture accurately. For that purpose, quantitative nuclear magnetic resonance (qNMR) spectroscopy is used to certify the individual mass fraction or content of the compounds (10, 11). qNMR enables direct measurement of an analyte against a primary reference as internal standard, thus resulting in a certified content value and traceability to the SI unit (12). Impurities do not affect the result of this certification step as long as one single NMR signal from each sample and the reference is pure enough for integration. After qNMR measurement of each compound, the compounds are dissolved in an appropriate solvent and diluted to the desired final concentrations. That gravimetric dilution step and the subsequent ampule filling is validated according to ISO 17034 requirements.
Before the development of a CRM is completed, stability and homogeneity of the compounds in the mixture must be determined. That cannot be carried out using qNMR because concentrations of the analytes in the solution are too low. Appropriate validated chromatographic methods for the control measurement are required. The homogeneity within and among randomly chosen ampules was tested using those methods. The same analytical methods were used to verify the stability of different compounds in the mixtures. Stability tests at storage temperature and at elevated temperatures were carried out using isotope-labeled analogs and isotope dilution MS (IDMS) whenever possible to achieve highest precision (13, 14).
In the stability studies, the concentration of each mixture compound was analyzed at certain times and results were compared with those of a frozen sample at –80 °C. Differences in concentration required incorporation of additional factor in the overall uncertainty of the certified mass fraction value (15). Figure 3 shows relative normalized concentrations of LC-MS E&L mix compounds over a period of nearly two years.
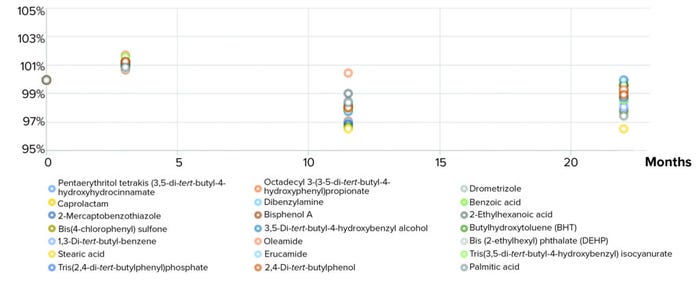
Figure 3: Stability test results of final extractables and leachables screening standard.
During stability testing of the first prototypes of the reference mixtures, some compounds were found to be unstable, including bisphenol F diglycidyl ether (BFDGE, CAS 2095-03-6), a common monomer for epoxy resins, for which the concentration decreased during storage. Investigations revealed that BFDGE reacted with 2-mercaptobenzothiazole (2-MBT), another compound in the mix, through a nucleophilic addition of the thiol-group of 2-MBT at the epoxide ring of BFDGE, leading to the formation of a secondary alcohol (Figure 4). As a consequence of that finding, BPFE was removed from the mixture. This example demonstrates that stability testing is indispensable and should be performed as well for in-house mix standards.
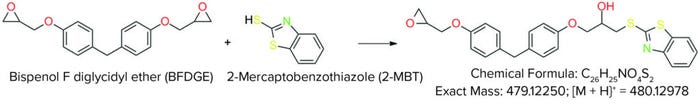
Figure 4: Reaction observed during stability test of one of the first test mixtures between 2-mercaptobenzothiazole and bisphenol F diglycidyl ether (BFDGE); as a consequence, BFDGE was removed from the final extractables and leachables screening standard.
Only after having controlled, verified, and determined homogeneity and stability of all mixture compounds, can a certificate of analysis be established for the entire mixture. That certificate includes all results and calculations from the certification process: homogeneity, stability testing, and certified concentrations with corresponding uncertainty ranges, traceability to the SI unit through the primary materials from the NMIs, and a fixed expiry date. The certificate can be downloaded from suppliers’ websites.
Use of Reference Standards
Method validation in analytical chemistry and ongoing QC depends on the availability of reference materials (16). The bias of an analytical procedure and its uncertainty also needs to be determined. For that purpose, matrix CRMs usually are used. Because matrix CRMs are not available for plastics, CRMs such as the developed E&L screening standards can be an alternative.
Method Validation: Reference mixtures are used in analytical chemistry for method validation. In principle, mixtures created in house can be used. However, a common requirement is to confirm such mixtures with an independent standard, ideally a CRM. Reference standards are then spiked at a certain level into a test samples with or without matrix —the standard addition method.
With such created test samples, the various performance parameters of an analytical method or the full analytical process can be assessed. That includes classical parameters such as linearity, selectivity, and LoD/LoQ estimations. Other essential parameters such as the bias of the analytical procedure also can be assessed to find systematic errors and to monitor random errors. Usually, that evaluation is performed by measuring available matrix CRMs (a cross-validation with an established reference or absolute method) or by spiking a reference sample (17). For extractables screening, the only option typically is spiking and generating a reference sample, because matrix CRMs and absolute methods are not available.
Complexity of a method validation depends on the analytical test. LC-HRMS analysis is the most challenging method in E&L studies. Table 2 shows typical validation parameters for that technique.
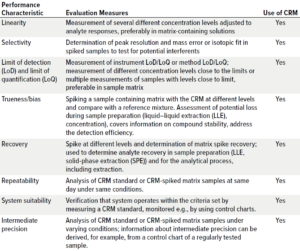
Table 2: Typical validation parameters for an analytical method that can be obtained by using a certified reference material (CRM) reference mixture; example is a liquid chromatography–high-resolution mass spectrometry (LC-HRMS) method.
QC is crucial to ensure validity of analytical results. Internal QC measures include analysis of system-suitability standards, reference materials, spiked samples, duplicates, and blank samples (18, 19). CRMs are used in QC such as for LC-MS screening. Self-defined reference materials such as polymer granules also can be used as alternatives (20). External QC includes, for example, proficiency testing to evaluate method performance between laboratories.
Control charts are among the most important tools for internal QC. They provide a visual indication of whether a parameter is in a state of control. They also are used to control an analytical process, verify calibrations, confirm correct working of a system in a system-suitability test (SST), and determine the uncertainty of a measurement. The simplest control chart displays the measured value with the mean value and defined limits, typically with warning (±2σ) and control (±3σ) limits. Control charts show potential trends, indicating, for example, whether a compound is unstable or unexpected systematic errors have occurred (e.g., if several consecutive measured values are above mean value).
Figure 5 is a typical mean-value control chart for a concentration of bisphenol A, a compound in the E&L screening standard for LC that is quantified with a calibration obtained with an in-house standard mixture. The concentration of BPA in the CRM was 10 µg/mL. The purpose of this study was to control the internal calibration by an independent reference mixture. That shows how a CRM can be used in routine QC to find systematic errors easily and to control random errors.
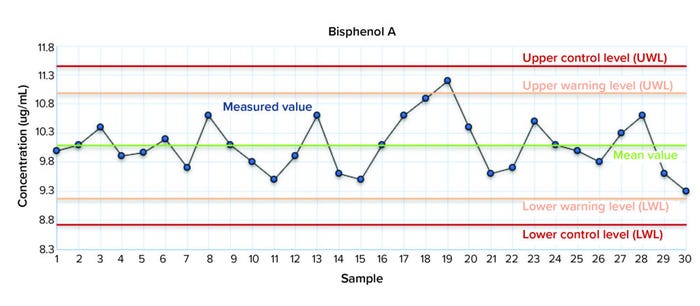
Figure 5: Mean value Shewhart control chart for bisphenol A of the independent CRM quantified by a calibration using an in-house standard mixture.
Measurement Details LC-MS
Two reference mixtures were developed and designed for GC-MS and LC-MS. GC-MS is a robust technique, with nearly standardized parameters and equipment. For E&L screening, the USP <1663> and the Product Quality Research Institute (PQRI) recommendations are excellent references for setting up a reasonable GC-MS method (3, 21). Official databases also are available, including the National Institute of Standards and Technology (NIST) Mass Spectral Library.
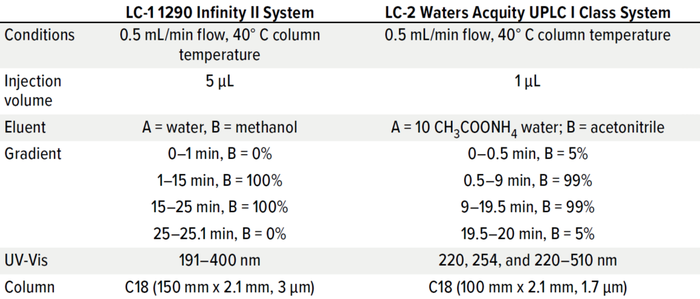
Table 3: Chromatographic conditions.
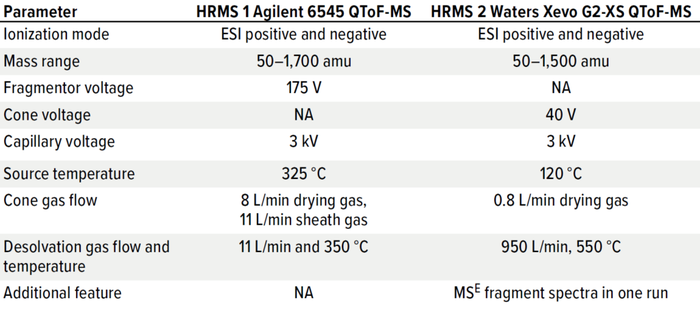
Table 4: Detection conditions of Agilent and Waters systems (HRMS = high resolution mass spectrometry, QToF = quadrupole time of flight, MS = mass spectrometry, MSE technology is a Waters brand).
For LC-MS systems, method parameters are complex, and the detection of multiple analytes is more difficult than for GC-MS. Different source designs and ionization methods can be implemented, matrix effects need to be considered, and different eluent systems and additives can be used. Tables 3 and 4 list analytical and detection parameters for systems from two suppliers. Such parameters could be used as starting points for setting up a new LC-MS method by using an E&L screening standard for LC.
Electrospray ionization (ESI) is the current technique for all LC-HRMS E&L screening. It is performed because most plastic additives feature an ionizable heteroatom and functionality. Compounds that are not ionized sufficiently by ESI might have a chromophore to make them accessible to UV detection, or they might be detectable by other orthogonal analytical methods routinely applied in E&L analysis. One example is alkanes, which are detected by GC-MS screening.
One drawback of ESI is the matrix dependence of the ionization efficiency, which can lead not only to ion suppression, but also to enhancement. In that context, using a CRM is an ideal way to check for such matrix effects by dilution within the matrix. For example, USP <665> recommends pH 3 or pH 10 buffers as extraction solvents. Dilution and calibration within those matrices provide information about whether and how well the different compound classes can be detected.
A simple transfer of parameters from one supplier’s system to another’s is not possible in LC-HRMS. Tables 3 and 4 show settings and details of the two systems that were used for analyzing the CRM mixture. Detectors included a quadrupole time-of-flight mass spectrometer (QTof-MS) for accurate mass measurements and a UV-Vis detector to detect weakly ionizable compounds. Detection wavelength should include λ = 220 nm, which represents a strong absorption of aromatic compounds because of a π → π* transition of phenyl groups. The UV signal can be used for semiquantitative estimation of concentration (e.g., of additives degradants or polymeric oligomers if a chromophore is unchanged compared with the parent compound) (22).
The setup detected most compounds, and the most prominent adducts were comparable between both systems with few exceptions. Only one compound, the aromatic compound 1,3-di-tert-butylbenzene, a degradant of the primary antioxidant tris(2,4-di-tert-butylphenyl)phosphite (e.g., Irgafos 168, from BASF), was not detected with MS because it lacks ionizable group. Such compounds are classic GC-MS analytes, but they also can be detected by means of their UV signal at 220 nm (Figure 6).
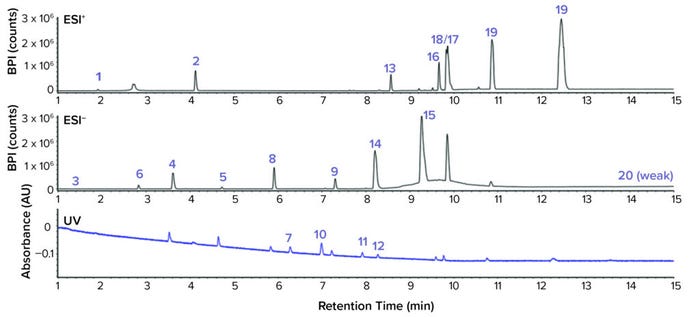
Figure 6: Extractables and leachables screening standard for LC system 2 at 1 μg/mL in isopropanol; base peak ion chromatogram for the ESI positive and negative ionization mode and UV chromatogram were recorded at 220 nm. All compounds of the CRM were detected with the combination of UV and MS detection.
The Agilent system readily detected 20 of the 21 compounds by ESI LC-MS. Detection was nearly identical for the Waters system, with the exclusion that no [M + H]+ adduct for bis(4-chlorophenyl) sulfone was observed; thus, 19 of 21 compound were detected. Typically for compounds with a high molecular weight (e.g., primary antioxidants), [M + NH4]+ adducts form because of the eluent additive. For the Agilent system, sodium adducts [M + Na]+ adducts were observed in ESI positive ionization mode because no further eluent additives were used (Table 5). Atmospheric pressure chemical ionization (APCI) also was tested with both systems. In general, the intensities of the peaks were significantly reduced compared with those using ESI. Some compounds showed almost no response at all and likely would escape an E&L screening. Based on those results, use of APCI as an additional ionization method did not provide further benefit compared with ESI. That is in accordance with screenings performed in environmental and water analytical chemistry in which ESI is the ionization method of choice.
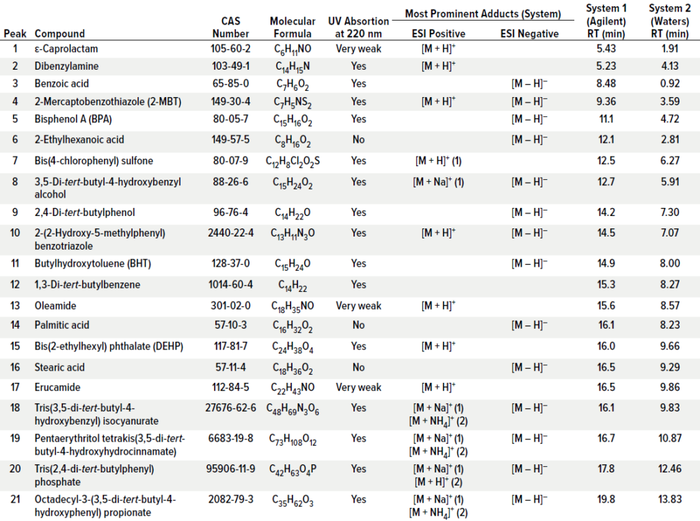
Table 5: Liquid-chromatography–high-resolution mass spectrometry (LC-HRMS) detection results for analytes of the extractables and leachables screening standard for liquid chromatography (ESI = electrospray ionization). The adducts depend on the systems used. For example, if a [M+H]+ adduct was only detected with system 1 (Agilent), then it is lablelled as “[M + H]+ (1).”
Effective Screening Methods
CRMs are an indispensable means in analytical chemistry to produce accurate and reliable measurements. They are used for method validation, as system-suitability standards, or for calibration.
No CRMs have been available for extractables screening of polymer materials and for leachables testing. Herein we provided details about the generation of an E&L screening standard for LC and GC systems by MilliporeSigma and how it can be used in routine laboratory work. We provided insight into quality measure for the setup of CRMs (e.g., traceability or absolute methods for content determination). We recommend that stability testing be performed for all self-prepared in-house mixtures to detect potential loss of analytes during storage.
We presented validation parameters for an LC-MS method for extractables screening and leachables target analysis in the context of a CRM. Control charts are simple but powerful tools, as shown in the example using a CRM reference mixture. We demonstrated the reliable identification and quantification of the E&L screening standard for LC for two LC-UV-MS systems. We showed which ionization mode should be used, what adducts are formed, and what compounds should be monitored by means of the UV signal. The system parameters presented might be used as a starting point to set up an analytical method covering a multitude of individual E&Ls of plastics materials and their respective chemical classes.
References
1 EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use. EudraLex Volume 4, Good Manufacturing Guidelines Part I, Chapter 3. European Commission: Brussels, Belgium, 2014; https://ec.europa.eu/health/system/files/2016-11/chapter_3_0.pdf.
2 Code of Federal Regulations, Title 21, Part 221.65 — Equipment Construction. US National Archives: Washington, DC, 2017; https://www.ecfr.gov/current/title-21/chapter-I/subchapter-C/part-211/subpart-D/section-211.65.
3 <1663> Assessment of Extractables Associated with Pharmaceutical Packaging/Delivery Systems. USP 41–NF 16. US Pharmacopeial Convention: Bethesda, MD, 2018: 7910–7924.
4 Kruve A, et al. Tutorial Review on Validation of Liquid Chromatography–Mass Spectrometry Methods: Part 1. Anal. Chim. Acta 870, 2015: 29–44; https://doi.org/10.1016/j.aca.2015.02.017.
5 Bradley E, Coulier L. An Investigation into the Reaction and Breakdown Products from Starting Substances Used to Produce Food Contact Plastics. Report FD07/01, Project Number A03054, Food Standards Agency. US Department of Agriculture: Washington, DC, 2007; https://www.nal.usda.gov/research-tools/food-safety-research-projects/investigation-reaction-and-breakdown-products-starting.
6 Jenke D. Compatibility of Pharmaceutical Solutions and Contact Materials: Safety Assessments of Extractables and Leachables for Pharmaceutical Products. John Wiley & Sons, Inc.: Hoboken, NJ, 2009.
7 Piringer OG, Baner AL. Plastic Packaging: Interactions with Food and Pharmaceuticals. Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2008; https://doi.org/10.1002/9783527621422.
8 ISO 17034: 2016: General Requirements for the Competence of Reference Material Producers. ISO: Geneva, Switzerland; https://www.iso.org/standard/29357.html.
9 ISO/IEC 17025: 2017: General Requirements for the Competence of Testing and Calibration Laboratories. ISO: Geneva, Switzerland; https://www.iso.org/publication/PUB100424.html.
10 Nelson MA, et al. A New Realization of SI for Organic Chemical Measurement: NIST PS1 Primary Standard for Quantitative NMR (Benzoic Acid). Anal. Chem. 90(17) 2018: 10510–10517; https://doi.org/10.1021/acs.analchem.8b02575.
11 Weber M, et al. Using High-Performance Quantitative NMR (HP-qNMR) for Certifying Traceable and Highly Accurate Purity Values of Organic Reference Materials with Uncertainties <0.1%. Accredit. Qual. Assur. 18(2) 2013: 91–98; https://doi.org/10.1007/s00769-012-0944-9.
12 Metrological Traceability in Analytical Measurement. 2nd ed. Ellison SLR, Williams A, Eds. Eurachem/Cooperation on International Traceability in Analytical Chemistry (CITAC), 2019.
13 Bedson P. Guidelines for Achieving High Accuracy in Isotope Dilution Mass Spectrometry (IDMS). The Royal Society of Chemistry, London, UK, 2002; https://doi.org/10.1039/9781847559302.
14 Vogl J, Pritzkow W. Isotope Dilution Mass Spectrometry — A Primary Method of Measurement and Its Role for RM Certification. J. Metrol. So. India 25(3) 2010: 135–164; https://doi.org/10.1007/s12647-010-0017-7.
15 Quantifying Uncertainty in Analytical Measurements. 3rd ed. Ellison SLR, Williams AJ, Eds. Eurachem/CITAC, 2018: https://www.eurachem.org/images/stories/Guides/pdf/QUAM2012_P1.pdf.
16 The Fitness for Purpose of Analytical Methods: A Laboratory Guide to Method Validation and Related Topics. 2nd ed. Eurachem, 1998; https://www.eurachem.org/images/stories/Guides/pdf/MV_guide_2nd_ed_EN.pdf.
17 Ellison, SLR, Barwick VJ, Farrant, TJD. Practical Statistics for the Analytical Scientist: A Bench Guide. The Royal Society of Chemistry, London, UK, 2009; https://doi.org/10.1039/9781847559555.
18 Emons H, Held A, Ulberth F. Reference Materials as Crucial Tools for Quality Assurance and Control in Food Analysis. Pure Appl. Chem. 78(1) 2006: 135–143; https://doi.org/10.1351/pac200678010135.
19 Guide to Quality in Analytical Chemistry: An Aid to Accreditation. Eurachem/CITAC 2016; https://www.eurachem.org/images/stories/Guides/pdf/Eurachem_CITAC_QAC_2016_EN.pdf.
20 ISO GUIDE 80:2014 Guidance for the In-House Preparation of Quality Control Materials (QCMs). ISO: Geneva, Switzerland; https://www.iso.org/standard/44313.html.
21 Norwood DL, et al. Safety Thresholds and Best Practices for Extractables and Leachables in Orally Inhaled and Nasal Drug Products. PQRI Parenteral and Opthalmic Drug Product Leachables and Extractables Working Group: Washington, DC, 2006; https://pqri.org/wp-content/uploads/2020/10/PQRI-PODP-Extractables-and-Leachables-Update_9Sept2020_FINAL.pdf.
22 Schaefer A, Ohm VA, Simat TJ. Migration from Can Coatings: Part 2 — Identification and Quantification of Migrating Cyclic Oligoesters Below 1,000 Da. Food Addit. Contam. 21(4) 2004: 377–389; https://doi.org/10.1080/02652030310001637939.
Corresponding author Roberto Menzel ([email protected]) is manager of extractables laboratory at Sartorius Stedim Biotech. Corresponding author Markus Obkircher ([email protected]) is head of R&D for reference materials and PT at MilliporeSigma. Matthias Nold is product manager reference materials at MilliporeSigma. Saskia Hähn is manager E&L at MilliporeSigma. Tanja Maier is scientist E&L at Sartorius Stedim Biotech. Marc Gemeinder is manager E&L at MilliporeSigma.
The Life Science business of Merck KgaA is operating as MilliporeSigma in the United States and Canada.
You May Also Like





