A Novel Seed-Train Process: Using High-Density Cell Banking, a Disposable Bioreactor, and Perfusion Technologies
A typical cell culture process begins with thawing of a cryopreserved cell-bank vial, followed by successive expansions into larger culture vessels such as shake flasks, spinners, Wave bags, and stirred bioreactors (1). When culture volume and cell density meet predetermined criteria, the culture is transferred to a production bioreactor in which cells continue to grow and express product. This approach presents several challenges.
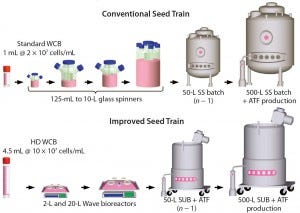
Figure 1: Comparing a conventional with an improved seed-train process for inoculation of a
500-L perfusion bioreactor; the new process uses high-density (HD) cell banking, single-use
technology, and high-density perfusion at the n – 1 stage to allow for high-density inoculation in
the production bioreactor. HD cell banking and disposables improve operational success by
reducing seed-train complexity and contamination potential. HD perfusion at the n – 1 stage allows
for HD inoculation of the production bioreactor, reducing the time to steady-state cell density by
4–5 days and increasing productivity by 10% for a 50-day run.
Shake flasks or spinners used in the initial stages require manual manipulations inside a laminar flow hood, making them vulnerable to contamination and operator error. Also, because a conventional cryopreserved cell-bank vial contains low cell numbers, the seed-train process is time consuming. It may take even longer and become more complex when production bioreactors are very large because additional large-volume culture vessels are required to support scale up to a production bioreactor. Finally, production bioreactors are usually inoculated at a density <0.5 × 106 viable cells/mL, so a necessary growth phase of 5–10 days offers little practical value because cell concentrations (and thus initial product yield) during that period will be low.
Previous efforts have shown that high-density (HD) cell banking can be an effective means to reduce the number of seed-train steps required and also improve operational success in seed-train processes. Tao et al. reported using HD cell banks containing 450 million viable cells/vial to directly inoculate a 20-L Wave bag (2). That study revealed that HD cell-bank use could eliminate several intermediate shake-flask expansion steps and reduce process times by up to nine days. Heidemann et al. combined HD cell banks with a single seed bioreactor capable of operating at several working volumes to reduce seed-train expansion duration by 60–70% (3).
Single-use technology offers numerous advantages over stainless steel systems, including enhanced facility flexibility, time savings in setup, cleaning, and sterilization, and reduced capital investment (4–6). Disposable Wave bioreactors from GE Healthcare Life Sciences are routinely used in seed-train expansion processes because they decrease contamination risk (typically requiring no manipulations in laminar-flow hoods). Also, because such bioreactors are disposable, no resources are needed for assembly, cleaning, or sterilization (7).
Industry interest in perfusion cell-culture processes has been increasing (8). Several companies have successfully used perfusion bioreactors in manufacturing commercial therapeutics (9). Perfusion processes can reach high cell densities in relatively small bioreactor volumes (10) and remove unstable product from cultures more quickly (11) than batch and fed-batch processes. Because of those and other advantages, perfusion is often considered in the context of production bioreactors rather than as an element of seed expansion.
Whitaker (12) and Heidemann (3) briefly mentioned using perfusion in seed expansion, but they did not discuss its potential to improve terminal seed-train stages, to reduce bioreactor size or steps, or to reduce the growth-phase duration for production bioreactors. Pohlscheidt (13) used a 3,000-L n – 1 perfusion bioreactor with an inclined cell settler to achieve n – 1 cell densities up to 15.8 × 106 viable cells/mL, decreasing cultivation times by 20% in a fed-batch production process. However, higher cell densities are hard to obtain with inclined settler devices because of perfusion rate limitations and long residence times for cells in suboptimal conditions (10).
Here we describe a novel seed-train process using HD cell banking, disposable Wave bioreactors, and Refine Technology’s Alternating Tangential Flow (ATF) perfusion cell culture technologies. Figure 1 compares an example of this process for a 500-L production perfusion bioreactor with a conventional seed-train process. Using the HD cell bank approach eliminates two intermediate spinner steps by allowing for direct inoculation into a 2-L Wave bioreactor. Replacing spinners (125 mL to 10 L in the figure) with Wave bioreactors (2 L and 20 L) significantly reduces the number of required manipulations in a laminar flow hood. That improves operational success by allowing operation under a “closed” system. Use of ATF perfusion technology at the n – 1 stage (50-L bioreactor with ATF perfusion device in the figure) also enables cell densities to be ≥50 × 106 viable cells/mL. That allows for an inoculation density of 5 × 106 viable cells/mL in the 500-L production bioreactor rather than 0.5 × 106 viable cells/mL in the conventional cell culture process. Higher seeding density helps reduce the 500-L production bioreactor growth phase by about five days, saving manufacturing plant time for a 500-L bioreactor operating for 50 days.
Materials and Methods
Cell Line and Media: All experiments used a commercially available chemically defined medium (Life Technologies) supplemented with 4 mM glutamine (Sigma-Aldrich). The Chinese hamster ovary (CHO) cell line is proprietary to Genzyme and produces a recombinant human enzyme.
Batch Seed-Train Culture in 2-L and 20-L Bags: We thawed an HD cell-bank vial (10 × 107 viable cells/mL, 4.5 mL/vial) into a 2-L Wave CellBag bioreactor (GE Healthcare Life Sciences) at a 1-L working volume. When viable cell density (VCD) in the 2-L Wave bag reached 3.0 × 106 viable cells/mL, we expanded the culture into a 20-L Wave CellBag bioreactor at a 7.5-L working volume. A model 20/50 EHTD Wave bioreactor system (GE Healthcare Life Sciences) served as the rocking platform for both the 2-L and 20-L bags, maintaining cultures at a temperature of 37 °C, a 16-rpm rocking speed, and a 7° rocking angle. A 20% O2 and 5% CO2 gas mixture flowed into the head space at a rate of 0.25 slpm.
Seed-Train n – 1 Perfusion Culture: We used a 15-L glass bioreactor (Broadley-James Corporation) equipped with an ATF4 perfusion device (Refine Technology) to mimic the n – 1 seed-train stage (50-L bioreactor in Figure 1). A 20-μm sintered sparger added oxygen to control dissolved oxygen (DO) at 40%, and a 1-mm drilled hole sparger added nitrogren to control the dissolved CO2 level <120-mmHg pressure. As needed, we added 0.5 M sodium carbonate (Na2CO3) to the culture for maintaining pH >6.85 and a 10% FoamAway antifoam solution (Life Technologies) to control the foam level.
We seeded a 15-L bioreactor at 10-L working volume with 0.5 × 106 viable cells/mL and operated the culture in batch mode until day 2, when perfusion began. Initially, an online capacitance sensor (Aber Instruments) and a DeltaV programmable logic controller (Emerson Process Management) controlled the cell-specific perfusion rate (CSPR) at 0.2 nL/cell/day. We capped the perfusion rate at four bioreactor working volumes per day (RV/day) after the VCD reached 20 × 106 viable cells/mL.
50-mL Spin Tube Batch Refeed Model for Inoculation Density Evaluation: We removed aliquots of the culture from the n – 1 seed-train bioreactor when its cell density reached 25 × 106, 50 × 106, and 100 × 106 viable cells/mL and subsequently inoculated those samples into 50-mL spin tubes (TPP Techno Plastic Products) at three different inoculation densities in triplicate: 0.5 × 106, 2.5 × 106, and 5.0 × 106 viable cells/mL in a working volume of 10 mL. Refeeds were performed once daily because continuous perfusion could not be performed at this small scale. Starting on day 1 we refed the cultures by removing the spin tubes from their incubator, spinning cells down at 1,100 rpm for five minutes, removing the supernatant, and then adding fresh media to resuspend the cells. That refeed strategy is designed to provide the same CSPR across different inoculation density conditions. A Multitron II shaking incubator (HT Infors) maintained all spin tubes at a temperature of 37 °C with a rocking rate of 160 rpm, a rocking angle of 45°, relative humidity of 80%, and CO2 concentration of 5%.
Production Bioreactor: To mimic the 500-L production bioreactor in Figure 1, we operated 15-L bioreactors (Broadley-James Corporation) with 10-L working volumes in perfusion mode using an ATF4 perfusion device (Refine Technology) with 0.2-μm polyethersulfone filters. A 20-μm sintered sparger added oxygen to control DO at 40%, and a 1-mm drilled hole sparger added nitrogen to maintain dissolved CO2 level <120-mmHg pressure. We maintained pH >6.85 by adding 0.5 M Na2CO3 and controlled foam levels by adding 10% FoamAway antifoam solution through a peristaltic pump.
One set of production bioreactors was inoculated at a low cell density (0.5 × 106 viable cells/mL) from a seed vessel operated in batch mode. Perfusion started on day 1 at 0.5 RV/day and increased daily by 0.5 RV/day until reaching a 2 RV/day perfusion rate. Another set of production reactors was inoculated at a high cell density (5.0 × 106 viable cells/mL) from an n – 1 15-L perfusion seed vessel when its density reached 50 × 106 viable cells/mL. Because of that high inoculation density, perfusion started at 2 RV/day immediately after inoculation. For all production bioreactors, an online capacitance sensor and bleed pump controlled the VCD at 40 × 106 viable cells/mL. All production bioreactors operated for 50 days.
Analytical Methods: We determined VCD using a ViCell XR cell viability analyzer (Beckman Coulter), measured pH and pCO2 off-line using a RAPIDLab blood gas analyzer (Siemens), and quantified protein productivity with a proprietary photometric enzymatic activity assay.
Results and Discussion
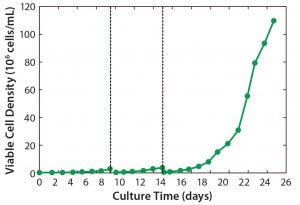
Figure 2: Viable cell density (VCD) profiles for a complete three-stage seed train
HD Cell Culture Using ATF Perfusion in the n – 1 Bioreactor: Figure 2 shows the VCD growth profile of our seed-train process including the two Wave bioreactor stages and the n – 1 stage. The 2-L and 20-L Wave stages lasted eight and five days, respectively. The n – 1 bioreactor operated for 12 days, reaching a final VCD of 110 × 106 viable cells/mL and >98% cell viability. VCD reached 50 × 106 cells/mL on day 9, which is the density required to inoculate a 500-L bioreactor at 5 × 106 viable cells/mL (from a 50-L n – 1 bioreactor).
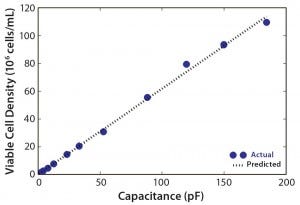
Figure 3: Viable cell density (VCD) as a function of capacitance for n – 1 bioreactor run
Initiated on day 2, n – 1 bioreactor perfusion was controlled to maintain a CSPR of 0.2 nL/cell/day by an online capacitance probe to automatically increase the feed rate as cell density rose. Success of such a perfusion-rate control strategy depends on the capacitance probe’s ability to accurately estimate VCD. Here, we used the strategy only until day 7 because the perfusion rate was capped at 4 RV/day, but the probe was able to accurately estimate VCD throughout the run to day 12, when 110 × 106 viable cells/mL was reached (Figure 3). Using control strategy during the cell growth phase allows much more accurate control of CSPR, which can be advantageous. Previous reports have shown that growth and productivity can be affected by a CSPR difference of 0.05–0.10 nL/cell/day (14). Our strategy also reduces the risk of incorrect or missed set-point changes because perfusion rate is automatically increased as the culture grows. In a stepwise approach, by contrast, operators must adjust the rate manually (15).
Evaluating the Effects on Cell Growth of n – 1 Split Density and Production Bioreactor Inoculation Density: Using a small-scale spin tube model, we determined how n – 1 cell density affected cell growth in the production bioreactor. We removed culture samples from an n – 1 bioreactor when cell densities were 25 × 106 viable cells/mL, 50 × 106 viable cells/mL, and 100 × 106 viable cells/mL, respectively. Then we split those into three aliquots diluted to 0.5 × 106, 2.5 × 106, or 5 × 106 viable cells/mL with fresh media for inoculation in triplicate into spin tubes. Daily refeeds began on day 1 at rates adjusted based on the inoculation density of the spin tubes. That allowed each inoculation density condition to experience the same CSPR at corresponding cell densities.

Figure 4: Viable cell density (VCD, solid line) and viability (dashed line) for
spin tubes inoculated at (a) 0.5 × 106 viable cells/mL, (b) 2.5 × 106 viable cells/mL, and (c) 5.0 × 106 viable cells/mL inoculation cell density; n – 1 stage final cell density is 25 × 106 (blue trends), 50 × 106 viable cells/mL (green trends), 100 × 106 cells/mL (red trends), respectively. Shaded areas represent ±1 standard deviation (n = 3).
Figure 4 shows cell count and viability profiles plotted to compare the n – 1 density conditions at each inoculation density. Because the maximum perfusion rate in the spin-tube model is 1 RV/day, we grew these cultures only up to 20 × 106 viable cells/mL to match their CSPR to that of the perfusion bioreactor model. We observed no growth differences among the n – 1 density conditions for all three inoculation densities tested. But the 100 × 106 viable cells/mL condition had a noticeably lower starting viability at all inoculation densities than at the other n – 1 densities.
To study the effects of n – 1 density and inoculation density on production bioreactor cell growth and productivity, we inoculated bioreactors at 5.0 × 106 viable cells/mL from an n – 1 bioreactor at 50 × 106 viable cells/mL and compared those with bioreactors inoculated at 0.5 × 106 viable cells/mL from an n – 1 bioreactor at 2.5 × 106 viable cells/mL. Figure 5 illustrates comparable cell growth for both conditions. Cell viability remained >90% for both inoculation conditions throughout the 50-day run. Figure 6 plots cumulative productivity against integrated VCD and indicates a similar specific production rate between conditions. Most notably, the steady-state cell density of 40 × 106 viable cells/mL (Figure 5) and the required titer for purification were reached four to five days sooner for the highest inoculation density condition.
Economic and Operational Advantages: Our seed-train process has both economic and operational advantages. For example, inoculating a 500-L perfusion bioreactor at 5.0 × 106 viable cells/mL reduces the growth phase duration by four to five days, increasing productivity by 10% for a 50-day run. Additionally, we have shown the n – 1 bioreactor could reach 100 × 106 viable cells/mL, theoretically inoculating a 500-L bioreactor at 10 × 106 viable cells/mL, which would reduce the growth phase duration by an additional five to six days.
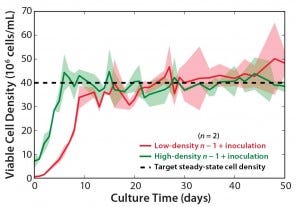
Figure 5: Viable cell density (VCD) profiles for 10-L production bioreactors inoculated at 0.5 × 106 viable cells/mL from an n – 1 bioreactor at 2
.5 × 106 viable cells/mL (n = 2) compared with 10-L production bioreactors inoculated at 5.0 × 106 viable cells/mL from an n – 1 bioreactor at
50 × 106 viable cells/mL (n = 2); dashed line represents the target viable cell density for steady-state operation, and shaded areas represent the range.
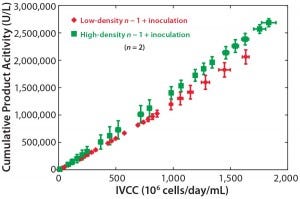
Figure 6: Cumulative product titer as a function of integral viable cell concentration for 10-L production bioreactors inoculated at 0.5 × 106 viable cells/mL from an n – 1 bioreactor at
2.5 × 106 viable cells/mL (red points) compared with 10-L production bioreactors inoculated at 5.0 × 106 viable cells/mL from an n – 1 bioreactor
at 50 × 106 viable cells/mL (green points); error bars represent the range.
Alternatively, our seed-train process can inoculate a production bioreactor operated in either batch or fed-batch mode. For a 21-day fed-batch cell culture process using a 500-L bioreactor, an inoculation density of 5.0 × 106 viable cells/mL could reduce the production bioreactor duration by 25%. Overall, that could allow for an additional five to six batches per year, increasing manufacturing productivity by 25–30%.
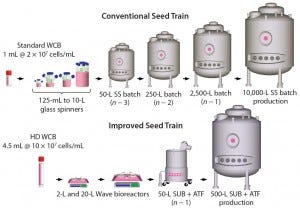
Figure 7: Comparing a conventional seed-train process to the future seed-train process for inoculation of a 10,000-L batch or fed-batch bioreactor; the future seed-train process uses high-density cell banking, disposables, and high-density perfusion at the n – 1 stage to eliminate two large-volume seed-train stages.
In most cases, batch and fed-batch processes use production bioreactors that are much larger than those used for perfusion processes, which requires several seed-train stages in large-scale stainless steel bioreactors. Figure 7 compares a conventional seed-train process used to inoculate a 10,000-L bioreactor with another process using a 50-L n – 1 bioreactor using an ATF perfusion device. The 50-L n – 1 bioreactor at 50–100 × 106 viable cells/mL can inoculate a 10,000-L bioreactor at 0.25-0.50 × 106 viable cells/mL, thus eliminating two intermediate seed-train stages. The benefit here is an increase in facility capacity use because no space and utilities are required for 250-L n – 2 and 2,500-L n – 1 bioreactors. Additional large savings could be realized in up-front capital expenditures.
A Simplified Seed Train
We have successfully demonstrated a novel seed-train cell culture process involving HD cell banking, single-use technology, and perfusion at the n – 1 bioreactor stage. This new procedure reduces the seed-train process complexity by decreasing the number of small-scale expansion stages and eliminating the need for labor-intensive operations such as cleaning, assembly, and sterilization. Manufacturing operations can rely on the same general seed-train process with a 50-L SUB at the n – 1 stage and a 500-L SUB at the production bioreactor stage.
Use of ATF perfusion at the n – 1 stage can help cultures achieve viable cell densities ≤100 × 106 viable cells/mL in 12 days without compromising culture growth characteristics after further expansion steps. Based on our work, a 500-L production bioreactor can be inoculated at a cell density of 5 × 106 viable cells/mL to reduce the time to steady-state cell density by four to five days and provide a 10% increase in the overall productivity of a 50-day run. This n – 1 perfusion technology can be adapted in several ways: An inoculation density of 10 × 106 viable cells/mL could be obtained in a 500-L bioreactor. For batch or fed-batch processes that require larger production bioreactors (e.g., 10,000-L), one or two stages can be eliminated from the expansion process by using a single 50-L perfusion bioreactor at the n – 1 stage.
References
1 Li F, et al. Current Therapeutic Antibody Production and Process Optimization. Bioprocessing J. September–October 2005: 1–8.
2 Tao Y, et al. Development and Implementation of a Perfusion-Based High Cell Density Cell Banking Process. Biotechnol. Progr. 27(3) 2011: 824–829.
3 Heidemann R, et al. A New Seed-Train Expansion Method for Recombinant Mammalian Cell Lines. Cytotechnol. 38(1–3) 2002: 99–108.
4 Smelko JP, et al. Performance of High Intensity Fed-Batch Mammalian Cell Cultures in Disposable Bioreactor Systems. Biotechnol. Progr. 27(5) 2011: 1358–1364.
5 Papavasileiou V, Siletti C, Petrides D. Systematic Evaluation of Single-Use Systems Using Process Simulation Tools. BioPharm Int. 21, March–April 2011: S16–S29.
6 Monge M, Sinclair A. Disposables Cost Contributions: A Sensitivity Analysis. BioPharm Int. 22, April 2009: 24–29.
7 Singh V. Disposable Bioreactor for Cell Culture Using Wave Induced Agitation. Cytotechnol. 30, 1999: 149–158.
8 Langer ES. Trends in Perfusion Bioreactors. BioProcess Int. 9(10) 2011: 18–22.
9 Chu L, Robinson DK. Industrial Choices for Protein Production By Large-Scale Cell Culture. Curr. Opin. Biotechnol. 12, 2001: 180–187.
10 Voisard D, et al. Potential of Cell Retention Techniques for Large-Scale High-Density Perfusion Culture of Suspended Mammalian Cells. Biotechnol. Bioeng. 82(7) 2003: 751–765.
11 Kadouri A, Spier RE. Some Myths and Message Concerning the Batch and Continuous Culture of Animal Cells. Cytotechnol. 24, 1997: 89–98.
12 Whitaker SC, Francis R, Siegel RC. Validation of a Continuously Perfused Cell Culture Process for Production of Monoclonal Antibody. ACS Symp Ser. 698, 1998: 28–43.
13 Pohlscheidt M, et al. Optimizing Capacity Utilization By Large Scale 3,000-L Perfusion in Seed Train Bioreactors. Biotechnol. Progr. 29(1) 2013: 222–229.
14 Dowd JE, et al. Optimization and Control of Perfusion Cultures Using a Viable Cell Probe and Cell Specific Perfusion Rates. Cytotechnol. 42(1) 2003: 35–45.
15 Kurkela R, Fraune E, Vihko P. Pilot-Scale Production of Murine Monoclonal Antibodies in Agitated, Ceramic-Matrix or Hollow-Fiber Cell Culture Systems. BioTechniques 15, 1993: 674–683.
Further Reading
Abu-Absi SF, et al. Defining Process Design Space for Monoclonal Antibody Cell Culture. Biotechnol. Bioeng. 106(6) 2010: 894–905.
Konstantinov KB, et al. The ‘‘Push-to-Low’’ Approach for Optimization of High-Density Perfusion Cultures of Animal Cells. Adv. Biochem. Eng. Biotechnol. 101, 2006: 75–98.
Woodside SM, Bowen BD, Piret JM. Mammalian Cell Retention Devices for Stirred Perfusion Bioreactors. Cytotechnol. 28(1) 1998: 163–175. •
Corresponding author Benjamin Wright is a process engineer, Neha Shah is a staff scientist I, Jin Yin is associate director, Mike Vrabel is a process engineer, Jason Walther is a staff scientist II, Seul-A Bae is a process engineer, and Konstantin Konstantinov is vice president, all in late-stage process development; Mike Bruninghaus is a manufacturing specialist supervisor in upstream purification operations; and Timothy Johnson is director of special projects and strategic support at Genzyme, a Sanofi Company, 200 Staples Drive, Framingham, MA 01702; 1-508-271-2080; [email protected]. Weichang Zhou is vice president of biologics process development at WuXi AppTec.
You May Also Like





