Product Quality Attribute Shifts in Perfusion Systems, Part 2: Elucidating Cellular Mechanisms
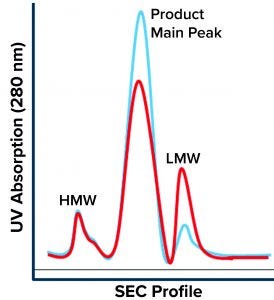
Figure 1: Size-exclusion chromatography (SEC) profile shows three main regions: high–molecular-weight (HMW) species, the product main peak, and low–molecular-weight (LMW) species. The blue chromatogram is a typical product profile; the red one is the atypical profile demonstrating high LMW with a lower product peak and no apparent change to the HMW peak.
Part 1 of this two-part report describes an investigation into the potential cause(s) and ways to control a product quality attribute (PQA) of a protein expressed in perfusion cell culture (1). The presence of low–molecular-weight (LMW) species following size-exclusion high-performance liquid chromatography (SEC-HPLC) is a protein quality attribute that can indicate an increase in truncated forms of the expressed protein and/or other LMW moieties. The expressed protein in this study is a heavily glycosylated recombinant glycoprotein (rGP) comprising two subunits: a 210-kDa heavy chain (HC) and an 80-kDa light chain (LC).
During a routine cell culture campaign, the SEC profile at the drug substance (DS) stage demonstrated a shift in LMW levels (Figure 1, in red). The root cause was determined to be an increase in cell residence time in the bioreactor system’s gravity-based cell-retention device that occurs as the perfusion culture campaign ages (Figure 2). Increased cell aggregation during such a campaign leads to an increased proportion of the cellular biomass residing in the cell-retention device. One way that aggregation can be detected is through monitoring of changes to cell discard (Figure 2). When cells accumulate in the retention device, fewer of them return to the bioreactor, which in turn reduces a culture’s demand for oxygen. Lower oxygen demand in the bioreactor reduces cell discard.
Previous work conducted at Bayer (not shown) has shown that a low cell-specific perfusion rate (CSPR) also increases LMW in SEC because it too can increase residence time in the cell-retention device. These insights have allowed us to establish a strategy to control LMW levels through process inputs (not shown). To enable the best means of controlling protein PQAs and/or prevent high levels of LMW, we needed to determine why longer residence time in the cell-retention device would cause an upward shift in LMW in SEC chromatograms. As described below, this understanding can provide important diagnostic tools to identify a high-LMW excursion early enough in a campaign to allow for timely preventative process controls.
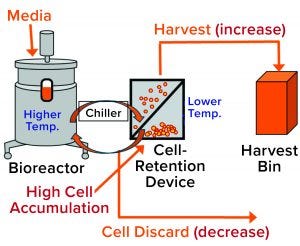
Figure 2: General setup of a perfusion cell culture system comprises a bioreactor (left), cell-retention device (center), and harvest bin (right). Cells from the bioreactor are chilled before entering into the retention device (2), where most of them settle by gravity and are returned to the bioreactor. Cell-depleted harvest is collected for further processing. Cell discard is controlled based on dissolved oxygen (DO) measurement from a probe located inside the bioreactor. Accumulation of cells in the retention device increases their presence in the harvest flow and decreases cell discard (red type in brackets).
Investigation
Because the cell-retention device is controlled at a much lower temperature than that of the bioreactor, cell accumulation in the device increases the proportion of the cellular biomass exposed to low temperature (2). Thus, we hypothesized that the high-LMW results might come from that prolonged exposure to low temperatures due to cell accumulation in the retention device (Figure 2). But why would cell exposure to low temperature affect LMW? Below, we postulate a cellular mechanism and provide experimental evidence demonstrating how exposure of cultured cells to lower temperatures could cause a transient increase in truncated forms of the expressed protein represented by the high-LMW region following SEC.
Site-Specific Cleavage: The discrete sharp peak of LMW following SEC (Figure 1) suggests site-specific cleavage of the rGP. That is unlike the nonspecific degradation that would manifest as a shallow peak area representing a broad distribution of truncated moieties. Site-specific cleavage implies the involvement of an enzyme that cleaves at a specific site to yield the molecular size of the species represented by the SEC LMW region. Specific enzymatic processing of glycoproteins does occur along their secretory pathway. Like most proteins, our nascent rGP is processed through the intracellular secretory machinery (Figure 3), where it goes through multiple posttranslational modifications (PTMs) as it is vesicularly trafficked through the endoplasmic reticulum (ER) and the Golgi compartments. Finally, it is secreted outside the cell into the culture medium from which it is harvested, isolated, and purified as a drug substance, then further processed into a drug product.
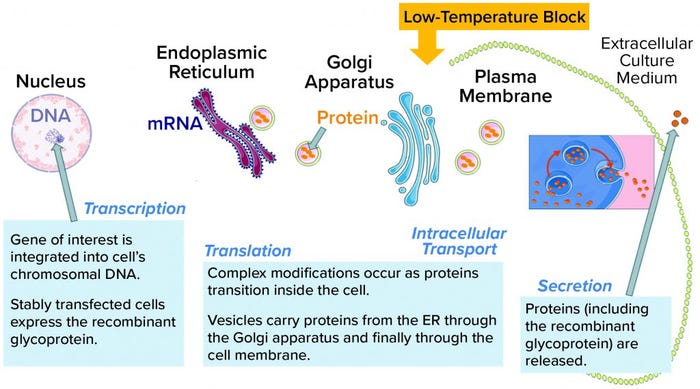
Figure 3: Glycoproteins are processed through the intracellular machinery of recombinant cells. A yellow arrow indicates the location of the temperature block at the trans-Golgi network (TGN); ER = endoplasmic reticulum.
The trans-Golgi network (TGN) is a key site where specific processing enzymes reside (3). It has been well established that different steps in the secretory pathway have distinct temperature requirements (4). A low temperature such as that in the cell-retention device prevents secretion from the TGN, as was reported for a model membrane glycoprotein (5). Note that this secretion block might not prevent terminal glycosylation (6). Furthermore, the low-temperature secretion block is reversible once cells have been returned to normal growth temperatures (as in a bioreactor). It is also common for different types of cargo proteins (see Table 1 in reference 6). All tested proteins accumulated in the TGN at low temperature and were released synchronously upon warming (6).
Low temperature affects secretion of our rGP. Because secretion of many glycoproteins is retarded at low temperature (4), we tested whether the low temperature that our cells are exposed to in the retention device could compromise rGP secretion. To do so, we established a secretion assay: Small-scale cultures were incubated at high (representing the bioreactor) or at low (representing the cell-retention device) temperatures for six hours, with samples collected at given time points. The samples were centrifuged, then protein was measured in the supernatants and in lysed cell pellets by densitometry following a Western blot (WB, not shown).
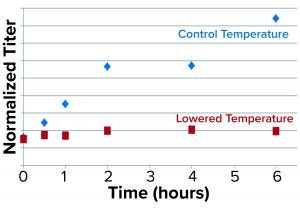
Figure 4: Results from the secretion assay show time-dependent blockage of secretion as represented by normalized rGP protein in the supernatant/pellet of low-temperature treated cultures relative to control.
We used antibodies specific for the rGP and normalized for dilutions to represent viable cell densities (VCDs) in the culture samples. As expected, we found that the rGP secretion — defined as the ratio of normalized rGP protein in the supernatant to that in the pellet — was found to be markedly reduced over time at lower temperatures (Figure 4).
Further experiments such as pulse/chase tracing by labeling the nascent glycoprotein and/or using inhibitors of protein synthesis followed by intracellular fractionation will be needed to account for reduced rGP biosynthesis (transcription and/or translation) at lower temperatures and to confirm/identify the specific intracellular site of the blockage in secretion. Although we could not account for the contribution of reduction in biosynthesis, the fast impact (observed already at the earliest time point of 30 minutes) of the low temperature is consistent with a blockage in intracellular secretion.
Nevertheless, the impact of that low temperature on secretion of rGP is consistent with a possibility that a larger proportion of the rGP is retained in the TGN and exposed to protease activity as a result of cell accumulation in the retention device.
Based on its amino acid sequence (not shown), the rGP expressed in this study contains two specific consensus cleavage sites for a known cellular endoprotease in the TGN that is involved in maturation of protein substrates along the secretory pathway. Preferential cleavage at one of those sites is expected to generate a ~120-kDa fragment. We expect that fragment to resolve under the LMW region following SEC (or later if further proteolysis should occur). The corresponding HC would be shortened to ~170 kDa (or less, if additional proteolysis occurs).
Conformational Differences: The ability of proteases to cleave proteins at particular sites harboring the specific amino-acid motif depends on the substrates’ tertiary structure as well as the amino acids immediately surrounding the cleavage site. Furthermore, low (or high) temperatures can influence a nascent protein’s folding, ultimately determining the glycoprotein’s conformation (and therefore, its susceptibility to proteolysis). At the reduced temperature, a few key changes might occur in our rGP-expressing cell culture to yield a higher proportion of the ~120-kDa fragment, thus giving rise to the elevated levels of LMW species. The experiment described above supports a low-temperature–induced inhibition of secretion that also could facilitate retention of the nascent glycoprotein at the TGN. It also suggests that a protein conformational change could make specific proteolytic sites more accessible to digestion by the protease (and/or a change in the protease’s sequence specificity). We explored this proposed mechanism further through experiments summarized below.
Process Analysis: It would be invaluable to know that a LMW excursion is imminent while changes to controlling culture parameters still can be made (e.g., relieving cell aggregation in the retention device to reduce cell exposure to lower temperature). However, by the time rGP substance is subjected to SEC and the potentially high LMW can be identified, it has been extensively purified (Figure 2 in reference 1) — far too late for corrective actions. Therefore, we explored the possibility of leveraging antibodies that are highly specific to the rGP and using prepurification of crude bioreactor-harvest samples to get an early read on the “intactness” of the rGP (the likelihood of a LMW excursion/increase) in line with the intracellular mechanistic insights described above.
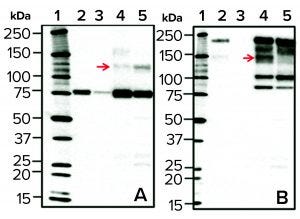
Figure 5: Western blots of cell culture batch samples from two bioreactors; normal (lane 4) and high LMW (lane 5) after rGP pull-down with anti-LC–coupled beads, probed with the anti-LC (panel a, left side) or anti-HC (panel b, right side) antibody. As a reference for the 80-kDa LC and the 210-kDa HC (in panels a and b, respectively), purified rGP was loaded in each blot at two concentrations (5 IU/mL in lane 2 and 1 IU/mL in lane 3). Lane 1 is a molecular weight standard. Top red arrows show an increase in the 120-kDa band (a) and a reduction in the 140-kDa band (b) in high LMW (lane 5) relative to normal (lane 4) bioreactors.
To that end, we subjected culture-harvest samples from normal (standard) bioreactors and those demonstrating high LMW to a “pull-down” procedure using beads coupled with anti-LC antibodies followed by WB with either anti-LC or anti-HC antibodies (Figure 5). Samples from bioreactor runs demonstrating high (above a maximum threshold) levels of LMW (Figure 1) showed an increase in a 120-KDa rGP LC-derived band and a concomitant reduction in a 140-kDa rGP HC-derived band (red arrows in Figures 5a and 5b, respectively). The 120-KDa fragment is detected under reducing sodium-dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) using an anti-LC antibody, suggesting that it consists of the 80-KDa LC with a 40-KDa portion, resulting in a higher molecular-weight LC-derived species. The 140-kDa band is a typically observed derivative of the HC.
Those results support a mechanism in which increased levels of the LMW can result from preferential cleavage at an alternative proteolytic site in the LC of the glycoprotein molecule, yielding the 120-KDa fragment. With the observed shifts in banding following WB analysis, this mechanism makes sense and is predicted based on the location of putative proteolytic sites on the amino-acid sequence of the expressed rGP (not shown). Enhanced exposure to the protease can occur when cells accumulate/aggregate in the cell-retention device and thus get exposed to much lower temperatures than they encounter in the bioreactor because low temperature retards the glycoprotein in the TGN (the intracellular compartment where the protease resides).
The observed shifts in WB banding profiles can serve as an early diagnostic or predictive indicator for cultures that demonstrate a presence of high-LMW variants. Using unpurified harvest samples in an antibody pull-down followed by WB has enabled detection of shifts in banding patterns indicative of changes to this PQA. Early identification of such changes saves the many days of delay otherwise to assess levels of LMW (because SEC requires using highly purified drug-substance samples) after the multiple ensuing purifications steps (Figure 2 in reference 1).
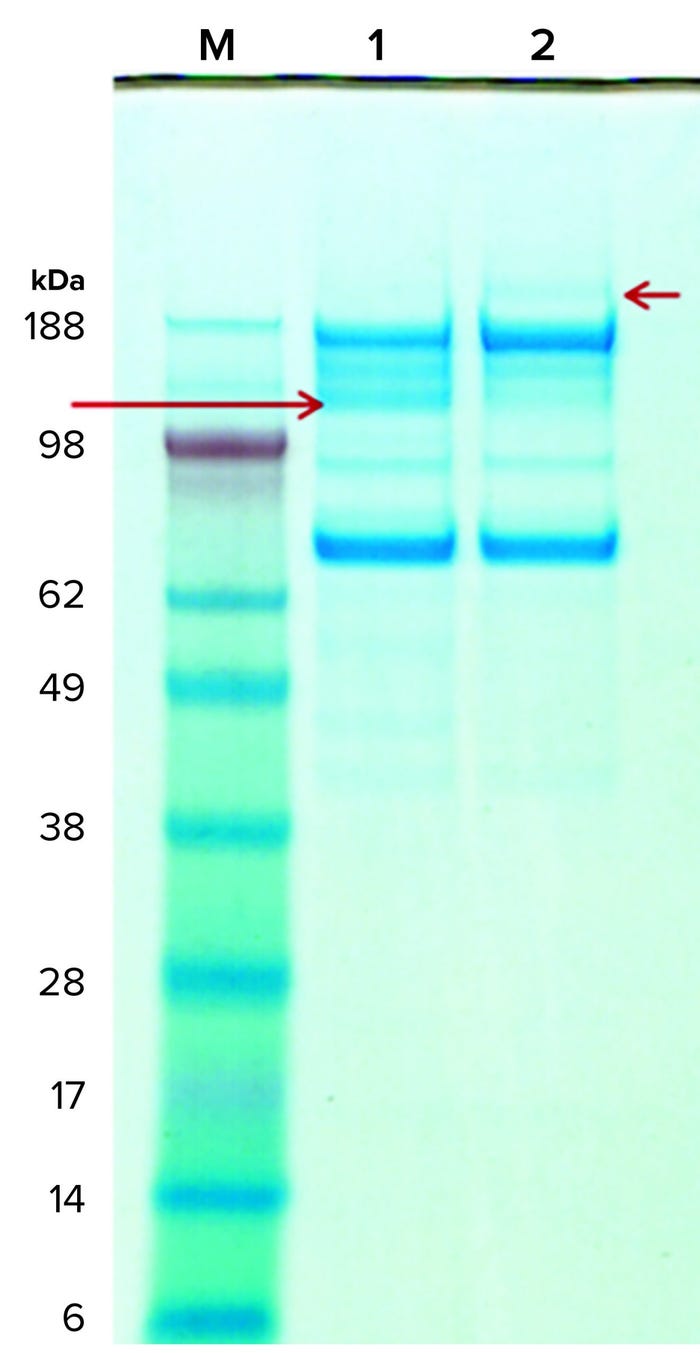
Figure 6: Coomassie-stained gels of purified rGP subjected (and not) to digestion by the implicated protease; lane M is the molecular-weight standard, lane 1 is the protease-digested rGP, and lane 2 is the mock incubated rGP (without the protease). A red arrow on the right points to the 210-kDa band in the untreated sample (lane 2); a red arrow on the left points to a 120-kDa band in the protease-treated sample (lane 1). The amount of protease used in the digestion is too small for detection by Coomassie stain, but a new band representing the protease was detected following silver-stain (not shown) and on SEC-HPLC (Figure 7).
Protease Digestion: Next, we tested whether our rGP can serve as a substrate for digestion by the implicated protease. In vitro digestion experiments showed that reconstituted purified rGP preparations are digested by the protease (Figure 6). Moreover, the ~210-kDa band (short red arrow, right side) representing the full-length HC of the rGP in the undigested samples collapses into a ~120-kDa band (long red arrow, left side) as predicted by the putative protease sites on the rGP’s amino-acid sequence. The additional fragments represent the expected 80-kDa fragment as well as additional (secondary) fragmentation, possibly facilitated through the structural change to the rGP molecule following the cleavage at the protease sites. Note that due to the heavy glycosylation of the rGP molecule, we expect the HC, LC, and fragments to migrate more slowly, showing an apparent higher than theoretical molecular weight. These results show that our rGP can serve as a substrate for the protease in vitro; they also support the existence of alternative proteolytic processing sites.
To investigate whether the 120-kDa protease digestion product reacting with anti LC antibodies (Figure 5, panel A) is the same species resolving under and increasing the levels of LMW, we subjected protease-digested purified rGP to SEC-HPLC. In scaled-up experiments we subjected protease-digested rGP, undigested rGP, and the protease alone (as a control) to SEC-HPLC. Aliquots from these reactions resolved on SDS-PAGE with silver staining to confirm digestion by the protease: Full-length rGP represented by the 210-kDa band collapsed into 120-kDa and 170-kDa species, as expected (Figure 6). Next, aliquots from the same reactions were subjected to SEC-HPLC.
Figure 7 shows the resulting SEC-HPLC chromatogram. Protease-digested rGP shows no apparent difference in HMW species or rGP product peak, and a notable decrease is observed in LMW species. However, one new peak following the LMW can be seen. Protease alone eluted at the LMW region, as expected based on its molecular weight.
Our results were reproduced in three independent experiments that did not exactly replicate the increase in LMW observed in the bioreactors with cell accumulation in the cell-retention device. Neither did they represent the increase in the 120-kDa band observed when high-LMW bioreactor samples and protease-digested rGP samples were resolved on SDS-PAGE. Still, the appearance of a new peak — indicating fragments even smaller than the LMW region 4 (red arrow in Figure 7) — suggests that further proteolysis or degradation of the 120-kDa species could have occurred during SEC-HPLC. That might be attributable to matrix effects or incompatibility between buffers used during proteolytic digestion and for running the HPLC. Matrix effects are supported as a reason by observed differences (most notably in the HMW region) compared with drug-substance chromatograms (Figure 1). The overlap between protease-digested rGP and undigested rGP in the product peak (intact rGP, Figure 7) indicates a minor impact of digestion by the protease to levels of the intact rGP, as observed by SEC-HPLC.
Proposed Cellular Mechanism
Data from our experiments suggest the following sequence of events:
The perfusion campaign is aging (possibly in combination with unknown triggers/variables).
Cell aggregation and accumulation in the cell-retention device possibly resulting from increasing cell density and/or reduced cell-specific perfusion rate.
Increased exposure of cultured cells to lower temperatures leads to intracellular accumulation and secretion-retardation of the rGP.
Alternative proteolytic processing (resulting in a distinct molecular-weight change in the LC) increases the presence of 120-kDa fragments over normal levels.
The 120-kDa fragment might coresolve at (and lead to an increase in) LMW following SEC-HPLC. Further fragmentation during SEC-HPLC could result in smaller LMW species. This fragment (and its derivatives) is likely to compete with the LC for binding to beads coated with anti-LC antibodies used in purification. That explains and is in agreement with an observed step-yield drop at that column purification step (Figure 6 in reference 1).
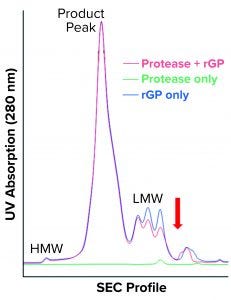
Figure 7: SEC-HPLC chromatogram shows protease-digested rGP (red), undigested rGP (blue) and protease only (green). HMW, product peak, and LMW are the three main regions; the red arrow points to the new shoulder observed in the protease-digested rGP sample.
As shown in Part 1 (1), cell accumulation in the retention device is correctable. It can lead to an increase in truncated rGP, as observed both following SEC-HPLC (presenting as high LMW) and SDS-PAGE WB (presenting as an increase in a 120-kDa fragment). Protease digestion replicated the observed increase in a 120-kDa band following SDS-PAGE. Although that did not replicate exactly the increase in LMW, an increase was observed in an area representing even smaller LMW species (red arrow, Figure 7), suggesting further proteolysis/fragmentation of the 120-kDa/LMW species. More work (digestion followed by fractionation) will be needed to optimize protease digestion in compatibility with SEC-HPLC, but that would require large amounts of purified rGP and enzyme/protease. Biochemical and microscopic methods (including imaging after immunostaining and/or subcellular fractionation techniques) could be used to explore further the impact of low temperature (and other critical culture variables) on intracellular trafficking and posttranslational modifications (e.g., glycosylation) of the rGP. Proteomics and metabolomics also could be used to gain an understanding of specific genes and pathways involved within the perfusion (or fed-batch) cultured host cells.
Process Control for Product Quality
In part 1 of this two-part report (1), we described the investigation process that has identified the root cause for high levels of LMW species by SEC: an increased cell-residence time in a cell-retention device as the perfusion cell culture campaign ages. In turn, accumulation of aggregated cells in the retention device (Figure 2) causes their prolonged exposure to low temperatures because relative residence time of cells in the device increases relative to the bioreactor. That is because the cell-retention device is controlled at a much lower temperature than the bioreactor (2). Cell residence time in the retention device can increase as a result of changes such as aggregation, CSPR reduction, and/or higher cell densities (2). Indeed, changes in control of the device were successful in resolving the increase in LMW.
The cellular mechanism supported by experimental data described herein sheds light on how low temperatures can cause alternative fragmentation of expressed rGPs by changing secretion, which in turn affects intracellular proteolytic activity. With this mechanistic understanding of the cellular level, we could devise a practical means of early detection and further avenues to control quality attributes of proteins expressed in perfusion-mode systems and prevent out-of-specification results from occurring.
Acknowledgments
We are grateful to the following Bayer (Berkeley) colleagues for their help in performing experiments: Gary Chiueh for running and troubleshooting bioreactors; and Zhenzhen Wang, Andreas Rosen, Chi-Fang Wu, and Khang Vo for performing proteolytic digestions, gel electrophoresis, and/or SEC-HPLC. Joel Cohen is acknowledged for fruitful discussions, coordination, and logistical help. We also thank our Bayer colleagues in Germany, Philipp Ellinger and Jens Burmeister, for performing Western blots and many late-night (early morning in California) teleconferences.
References
1 Pathange LP, Shimoni Y, Srinivasan V. Product Quality Attribute Shifts in Perfusion Systems, Part 1: Identifying Shifts When They Occur. BioProcess Int. 18(9) 2020: https://bioprocessintl.com/upstream-processing/perfusion-cell-culture/product-quality-attribute-shifts-in-perfusion-cell-culture-systems-part-1-identifying-shifts-when-they-occur.
2 Shimoni Y, et al. Reducing Variability in Cell-Specific Productivity in Perfusion Culture. BioProcess Int. 16(1–2) 2018: 22–31; https://bioprocessintl.com/upstream-processing/perfusion-cell-culture/reducing-variability-in-cell-specific-productivity-in-perfusion-culture-a-case-study.
3 Steiner DF, et al. Golgi/Granule Processing of Peptide Hormone and Neuropeptide Precursors: A Minireview. J. Cell. Biochem. 24, 1984: 121–130; https://doi.org/10.1002/jcb.240240204.
4 Saraste J, et al. Temperature-Sensitive Steps in the Transport of Secretory Proteins Through the Golgi Complex in Exocrine Pancreatic Cells. Proc. Natl. Acad. Sci. 83, 1986: 6425–6429; https://doi.org/10.1073/pnas.83.17.6425.
5 Matlin KS, Simons K. Reduced Temperature Prevents Transfer of a Membrane Glycoprotein to the Cell Surface But Does Not Prevent Terminal Glycosylation. Cell 34, 1983: 233–243; https://doi.org/10.1016/0092-8674(83)90154-x.
6 Traub LM, Kornfeld S. The Trans-Golgi Network: A Late Secretory Sorting Station. Curr. Opin. Cell Biol. 9, 1997: 527–533; https://doi.org/10.1016/s0955-0674(97)80029-4.
Corresponding author Yuval Shimoni is principal quality assurance specialist in the quality risk and knowledge management group; Prasad Pathange is director, and Venkatesh Srinivasan is vice president of manufacturing sciences and technology at Bayer US LLC, 800 Dwight Way, Berkeley, CA 94710; [email protected].
You May Also Like





