Partnerships in Immunotherapy for the Future of Cancer Treatment
February 14, 2019

ADOBE STOCK (HTPPS://STOCK.ADOBE.COM)
Immunotherapy seeks to harness the power of our human immune system to fight disease. In this rapidly evolving field, collaboration among different stakeholders is essential to bringing new treatments to market. Patient advocacy groups, researchers, hospitals, manufacturers, and government entities all are working together to translate promising new research into life-saving products. Types of immunotherapy include monoclonal antibodies (MAbs) and antibody derivatives, checkpoint inhibitors (immune-modulating proteins), cancer vaccines, T-cell therapies, and cytokines — so the approach involves a range of product modalities: proteins, gene therapies, and cell therapies. Thus, their manufacturing issues run the gamut of bioprocessing. Product development involves specialized clinical trials and efficacy test methods, however.
With the complex nature of human immune systems and cancer biology, novel collaborations are needed to turn exciting research into viable treatments. For example, private research institutions collaborate with public entities to gain funding and resources that facilitate their advancement of new therapies. As clinical advancements are made and new treatments or potential cures are developed, the inevitable question of affordability arises. New services and technologies are needed to provide a wide range of patients with improved access to immunotherapies. Novel pricing models may be necessary.
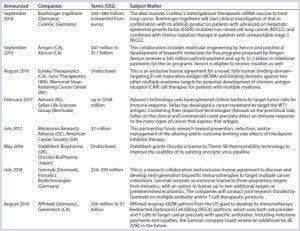
Table 1: Immunotherapy partnership examples
Immunotherapies aren’t only for cancer treatment (also, e.g., allergies, infectious disease), but that is so far the largest area of their development. The US government’s “Cancer Moonshot” program (https://www.cancer.gov/research/key-initiatives/moonshot-cancer-initiative) has helped to accelerate such endeavors. A number of partnerships have been initiated (Table 1), many involving Moonshot money, among large and small pharmaceutical companies, research institutions, contract service providers, and disease-focused patient advocacy groups. Each organization has something unique to offer and seeks out particular capabilities in potential partners.
The Parker Institute for Cancer Immunotherapy (San Francisco, www.parkerici.org) is one such entity for which collaboration is key to driving the best science. Cancer centers partner with both industry and nonprofit organizations with a goal of elevating cancer immunotherapy research projects and clinical trials. In a video on the Parker Institute’s website, Ilan Zipkin (vice president of business development) says, “When we talk about business development at the Parker Institute, we’re talking about collaborations and partnerships — with our member researchers, other nonprofits, and especially companies across the entire range of the biotech and pharma industry. Working with these partners gives us the opportunity to push the boundaries in cancer therapy in innovative ways that wouldn’t happen without the collaborative effort of multiple parties coming together through the Parker Institute.”
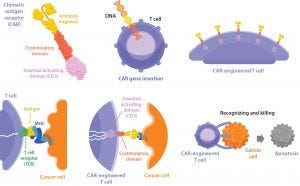
Figure 1: Chimeric antigen receptor (CAR) T cell therapy (HTTPS://STOCK.ADOBE.COM)
The Cell Therapy Perspective
Many immunotherapy approaches are based in cell and/or gene therapy modalities. T-cell therapies based on chimeric antigen receptor (CAR) technology are just one example (Figure 1). At KNect365’s 2018 Cell and Gene Therapy Bioprocessing conference — part of Biotech Week Boston — a number of companies involved in immunotherapy were represented in the program. In a special workshop on partnering, several leaders in the field shared their ideas of best practices in working with others to reach shared goals.
For example, Chris Gemmiti (vice president of operations at Sentien Biotechnologies) presented on “Phase-Appropriate Manufacturing: Internal vs. External Manufacturing During Company Growth.” He compared the benefits and drawbacks of outsourced manufacturing for up-and-coming regenerative medicine companies. He said that they must develop their own depth of understanding about their products and processes. Robust characterization is the foundation of that. Internal manufacturing maximizes control, but it comes at a significant cost driven by infrastructure and people. Strategic fit between client and contract manufacturing organization (CMO) is key because in the biopharmaceutical industry, outsourcing is a long-term relationship. And there is no single best solution for everyone. Each company will evolve on its own subject to development milestones and funding along the way. Gemmiti emphasized a strategy of slowly shifting from external services to internal capabilities over time as a small company grows.
Wouter Van’t Hof (cord blood bank director at the Cleveland Cord Blood Center) offered an interesting proposal in “Capabilities of Blood Centers and Banks: Could They Be the CMOs of the Future?” He described state-of-the-art processing facilities and procedures used in the blood industry and how they could translate to work for cell therapy purposes. Umbilical cord blood facilities already must comply with good manufacturing practice (GMP) regulations.
Linda Kelley (director of the cell therapy facility at Moffitt Cancer Center) spoke on “Leveraging Academic and Commercial Contract Manufacturing Organizations to Facilitate Accelerated FDA Approvals.” She described how the 21st Century Cures Act and an FDA mandate for accelerating the approval process for cellular therapies has changed the approach to GMP manufacturing of such products for early phase clinical trials. Moffitt has embraced an academic and industry partnership to facilitate multicenter clinical trials using tumor infiltrating lymphocytes (TILs) and CAR-T cells for liquid and solid tumors. “Through a combination of process improvements intended to shorten product manufacturing time,” Kelley said, “as well as innovative implementation of GMP practices, we have maximized the contribution of academic and contract manufacturing organizations to support multiple on-going trials intended to receive accelerated FDA approval for a new cellular therapy.”
A PACT with the Government
In October 2017, the US National Institutes of Health announced an ambitious US$215 million, five-year public–private research collaboration effort called the Partnership for Accelerating Cancer Therapies (PACT). Initially the participants will focus on identifying, developing, and validating robust biomarkers to advance new immunotherapy treatments. Eleven leading biopharmaceutical companies are involved: AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Genentech, Gilead Sciences, GlaxoSmithKline, Janssen Pharmaceutical, Novartis, and Pfizer.
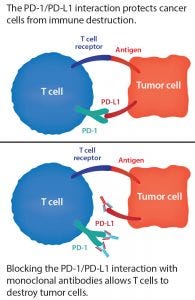
Figure 2: The PD-1/PD-L1 blockade
Immunotherapies have seen dramatic success in certain cancer cases and are the focus of intense investment, but they don’t work for all patients. For instance, PD-1 and PD-L1 checkpoint inhibitors are MAbs that block proteins present on the surface of T and tumor cells (Figure 2). One example is Merck’s Keytruda (pembrolizumab), which is FDA approved to treat certain cancers of the lungs, skin, stomach, cervix, bladder, head and neck, and lymph nodes. Such checkpoint inhibitors offer hope to some patients for whom chemotherapy alone has not been enough. And these treatments can boost the survival rate of patients who take them in combination with chemotherapy or radiation. But that boost is by degree only — e.g., improving a 50% survival rate to 66%. With the immunotherapy alone, the survival rate can be 20–40%. But the question then becomes, “What makes those survivors special?” Development and standardization of biomarkers will help developers understand how and why immunotherapies help some patients and learn how to predict those responses.
“We have seen dramatic responses from immunotherapy, often eradicating cancer completely for some cancer patients,” said NIH director Francis S. Collins in a PACT press release. “We need to bring that kind of success — and hope — for more people and more types of cancers, and we need to do it quickly. A systematic approach like PACT will help us to achieve success faster.”
According to NIH, the US$215 million program will facilitate systematic and uniform clinical testing of biomarkers to advance knowledge of the mechanisms of response and resistance to cancer therapy. Research conducted will integrate immune and other related oncology biomarkers into clinical trials by defining a set of standardized biomarkers to be tested across the range of studies. This approach should allow for consistent data generation through uniform and harmonized assays that support reproducibility, comparability of data across trials, and discovery and validation of new biomarkers. PACT also will facilitate information sharing for improved coordination of clinical efforts and alignment of investigative approaches, while reducing duplication and enabling more high-quality trials to be conducted.
“A scientific and organizational challenge this complex cannot be addressed effectively by any one organization acting alone,” said Maria C. Freire, president and executive director of the Foundation for the National Institutes of Health (FNIH), which is managing the program. “It requires the energies and resources of public and private partners working in close collaboration.”
NIH’s National Cancer Institute (NCI) has awarded cooperative agreements to support four cancer immune monitoring and analysis centers (CIMACs) and a cancer immunologic data commons (CIDC) with a total of $53.6 million in funding over five years. Those will form a network of laboratory centers to support adult and pediatric immunotherapy trials. CIMAC researchers will perform deep-tumor and immune profiling, with the resulting data collected in the CIDC database. This network also provides a foundation for laboratory, assay development, and database functions required by PACT.
“NCI’s long-term support for basic and translational research in immunotherapy paved the way for recent dramatic clinical successes in this area,” said Douglas R. Lowy (acting director of NCI). “This partnership — and the data the partners have committed to making publicly accessible to the broader research community — will facilitate our continued progress in helping to find the cancer treatments that benefit the greatest number of patients.”
The NCI cooperative agreements have been awarded to Dana-Farber Cancer Institute (Boston), Stanford Cancer Institute (Stanford, CA), Precision Immunology Institute and the Tisch Cancer Institute at Icahn School of Medicine (Mount Sinai, NY), and the MD Anderson Cancer Center at the University of Texas (Houston).
Some PACT Program Participants Say . . . |
“Given the significant unmet needs in oncology, it’s critical that we work together to drive clinical research and development. Tackling the toughest challenges is going to take all of us: scientists, physicians, industry peers, patient groups, and patients.” — Tom Hudson (AbbVie) |
“Immunooncology is changing the way cancer is treated, but we are still limited in our ability to identify responding patients. A huge step forward in the field will be to identify relevant biomarkers for patient selection and develop standardized assays for the analysis of immune parameters in treated patients.” — Michel Pairet (Boehringer Ingelheim) |
“We are in an unparalleled time of medical advances, which can continue to improve patients’ lives, decrease mortality, and reduce the burden on healthcare systems.” —Mark J. Alles (Celgene) |
“When industry, academia, and government work together to share scientific knowledge and new technologies, we have a chance to make significant strides in the fight against cancer and to ultimately help more people.” — Edith Perez (Genentech/Roche and the Mayo Clinic) |
“We are at the beginning of a new era of personalized cancer treatment with engineered cell therapies. PACT research has the potential to advance understanding of the field and demonstrate yet again the importance of public–private partnership in driving advances to address patients’ unmet medical needs.” — Kacy Hutchison (Gilead Sciences) |
“By harmonizing the way we use biomarker tools, we can reduce data heterogeneity and accelerate development of immunotherapies.” —Axel Hoos (GlaxoSmithKline and PACT cochair) |
“We aim to make a meaningful difference in the way we test, validate, and share vital information about biomarkers.” —Peter Lebowitz (Janssen Pharmaceuticals) |
“Ensuring that cancer patients derive the maximum possible benefit from advances in immunotherapies is at the core of Pfizer’s R&D efforts. We are proud to support the PACT and its urgent mission to work with the health innovation ecosystem to advance the impact of the latest cancer advances to a larger group of patients.” —Robert T. Abraham (Pfizer Worldwide Research and Development) |
SOURCE: HTTPS://WWW.NIH.GOV/NEWS-EVENTS/ WHAT-PACT-PARTNERS-ARE-SAYING |
Working Together Is the Way Forward
Biopharmaceuticals are a particularly complex expression of medicine — and immunotherapies arguably even more so. As treatments, they often also need “partners” of a kind themselves: e.g., radiation/radiotherapies, traditional MAbs, and chemotherapies. “The most effective therapies are combinations of different products,” INmune Bio CEO Raymond Tesi told me in late 2018. “Few companies are developing multiple therapies that work synergistically. Consequently, it is rare for one biopharmaceutical company to have all the elements needed for an effective combination-therapy strategy. As a result, most companies need to form partnerships with others in the field.” And outsourcing is essential. “The modern clinical-stage biotech company is small, lean, and virtual. It is financially ruinous to ‘own’ an expertise that is needed during only one part of the development cycle.” Government, commercial, medical, and research organizations must work together to advance the cause of immunotherapy for cancer — and beyond.
A Conversation with Consultant Jayson Slotnik |
Jayson Slotnik is a principal and founding member of Health Policy Strategies, Inc. (Washington, DC) — a consulting firm representing innovative biotechnology, pharmaceutical, medical device, and diagnostic companies. After Biotech Week Boston, we discussed partnering in immunotherapy development. |
What is it about immunotherapy that makes it difficult for a single company to go it alone? The manufacturing and distribution model for cell, gene, and immunotherapies is much more expensive due to the nature of living cells and time from manufacturing completion to patient administration. Scale-up is very expensive, so no small to midsize single company can “go at it alone.” In addition, because there are only a few marketed products, the models for manufacturing and distribution are not yet well founded. Companies may think that they have many options or that they can and should try different ideas. This adds to the expense both in terms of time and money. |
What challenges do immunotherapy developers face in financing and product launch? I believe the main challenge it is the unknown of future costs related to manufacturing and regulatory environments. Partnerships of the right kind can dramatically reduce uncertainty because of the infused capital and expertise (e.g., in the Gilead acquisition of Kite). Successful partnerships are those that bring technical expertise along with capital to efficiently gain regulatory approval and establish a successful commercialization strategy. |
What are the unique issues related to oncology treatment when it comes to reimbursement? Issues related to reimbursement depend on the site of care and mode of administration. Those factors dictate the reimbursement methodology, which in turn dictates the commercial and market access strategy. Developers should understand early on in product development that each site of care has a unique reimbursement methodology. That way, they can design clinical trials that will generate the right data to demonstrate the value proposition to payers in each site of care. In addition, some sites reimburse lump sums of money through a prospective payment system. Therefore, developers must be aware of different price points depending on the lump sums generated by the disease being treated. |
When we talk about the product lifecycle, often people focus on the period beginning with product licensure. What happens here with immunotherapies, and how is that expected to change in the future? Partnerships in the immunotherapy space occur well before licensure for many of the reasons stated above. Smaller product developers need the expertise and money from larger companies to achieve final regulatory approval and to manufacture and distribute the product on a large scale. |
How can partners help one another once their products are on the market? Once an immunotherapy is on the market, partnerships can be very helpful in educating prescribers, payers, and patient communities on the value proposition of that therapy and on when and how it should be used. |
One thing we see more in the industry these days is partnership between the users and suppliers of biotechnology equipment, instruments, and so on. This seems to be tied to innovation. What aspects of immunotherapy product development are most in need of such innovation now? It seems to me that manufacturing and distribution are in need of innovation. The current model was developed for pills and monoclonal antibodies (MAbs), both of which can be mass produced for a broad range of patients. Given the more personal nature of immunotherapies, this model does not fit. I suspect that companies will emerge to specialize in these functions much in the way that specialty pharmacies and distributors emerged for certain classes of drugs. |
Cheryl Scott is cofounder and senior technical editor of BioProcess International, PO Box 70, Dexter, OR 97431; 1-646-957-8879; [email protected].
You May Also Like





