Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
October 1, 2010
Part 1 of this two-part article introduced the need to reduce the environmental footprint of bioprocesses and evaluated the impact of solid-waste disposal. Part 2 continues by describing the effects of the remaining elements of the bioprocess footprint: wastewater, electricity, and air emissions.
Wastewater
Process Waste Streams: Generally, raw materials to produce and purify biopharmaceuticals fall into one of the following categories: inorganic/organic salts, sugars/polyols, trace elements, vitamins, amino acids, surface active agents, or complex (undefined) ingredients (Table 1). Most bioreactor media components are either life-supporting or life-promoting and thus likely to have a low intrinsic toxicity. However, when used at concentrations necessary to achieve commercially viable processes, these compounds can be toxic to aquatic life. Overall guidance for fermentation broth and downstream isolation component selection during early process development is best based on each component’s concentration and potential hazard. Bioprocess scientists benefit from understanding acceptable limits for raw material components (most specifically trace metals) acceptable for sewering discharges as processes are improved. Although these boundaries in turn depend on local waste flow conditions and wastewater permit limits, several general considerations can be assembled to guide the selection and use of raw materials for culture media and buffers.
PRODUCT FOCUS: ALL BIOLOGICS
PROCESS FOCUS: UPSTREAM AND DOWNSTREAM
WHO SHOULD READ: PROCESS DEVELOPMENT AND MANUFACTURING
KEYWORDS: SINGLE-USE, DISPOSABLES, SUSTAINABILITY, ENVIRONMENTAL IMPACT
LEVEL: INTERMEDIATE

Table 1: Typical media concentration ranges (not all ingredients are present in each medium)
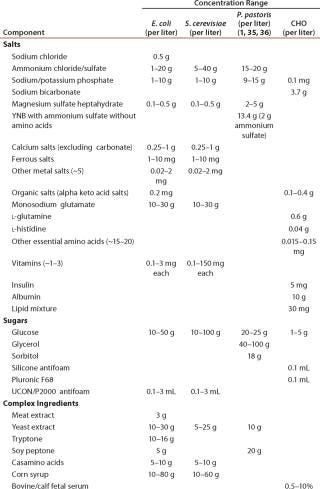
Table 1: Typical media concentration ranges (not all ingredients are present in each medium) ()
Production Operations: Table 1 shows typical concentrations for initial fermentation growth media components. Trace elements, including metals, are either added directly to media or are indirectly present in complex nutrients or multicomponent media powders. When total initial media concentrations of trace elements are compared for various host systems, Escherichia coli and Saccharomyces system values are notably higher than Chinese hamster ovary (CHO) and Pichia system values (Table 2). For E. coli, totals reach ~25 mg/L of which >75% is Fe and therefore can be an environmental concern (Table 3). Feed solutions can contain either whole media or only limiting nutrients, but in either case they often contain trace metals either directly or indirectly. For intensified processes, accumulated component feed amounts and the eventual location of component accumulation must be considered. For example, small amounts of carbon, nitrogen, and oxygen components are incorporated into the desired biopharmaceutical products, but larger amounts are emitted as by-products, biomass, and off-gas.
Table 2: Typical levels of trace element compositions in initial batched medium for various types of cultures (not all elements present in each media for each host system)Table 3: Toxicity data from literature
During fed-batch operation for process intensification, trace metals accumulate intracellularly but also can accumulate extracellularly if the nutrient feed composition is unbalanced. Fed-batch additions to Pichia fermentations include glucose (500–600 g/L), glycerol (500–750 g/L), and salt solutions containing Cu, I, Mn, B, Fe, Zn, Co, Mo, B, or I and other nonelement items (1). Table 4 shows unbalance for a Pichia fermentation, in which extracellular trace metal increases relative to uninoculated medium are significant: Mo (threefold), Fe (twofold), and Cu (>30-fold). The accumulation of Cu to 3.7 ppm (mg/L) at harvest exceeds the toxicity limit by at least one order of magnitude, becoming a more serious problem than the initial uninoculated levels (Table 3).
Table 4: Trace element distribution for BMGY fed-batch Pichia pastoris process at various stages
Toxicity testing data are highly dependent on the organism required for the acute toxicity evaluation, which differs depending on a biopharmaceutical facility’s physical location. These differences are important to understand for companies executing processes in multiple locations including outsourcing vendors. At one Merck location (Rahway, NJ), for example, toxicity tests use mysid shrimp (Americamysis bahia) to indicate effects on the nearby salt-water discharge environment. At a second Merck location (West Point, PA), toxicity tests use Daphnia magna to indicate effects on the nearby fresh-water discharge environment. Differences in toxicity are evident depending on the organism used, with the fresh-water organism (Daphnia) often being 10-fold more sensitive to trace metals (Table 3). Regardless of location, some minimal toxicity assessment before sewering is prudent, which may require actual aquatic tests if previous data do not exist.
Relative and combined toxicities of toxic components, particularly trace metals, also are important considerations. Table 3 values are based on individual component testing and should be supplemented by testing a composite sample of the entire stream under evaluation. Combined (synergistic) toxicities of metals are evaluated, but not necessarily for commonly observed bioprocess concentrations and elements, which typically do not use Hg, Cd, Pb, and Ni (2). For each treatment plant, daily municipal sewering limits for certain metals and other components (e.g., CN) are established based on concentration, stream volume, and expected dilution/augmentation when mixed with other facility or site wastes flowing to the treatment plant. (It is not permissible to dilute waste upon disposal to achieve sewering limits.)
Generally trace minerals are present at a level of 10–4 M (0.1 mM) in uninoculated medium (3). Calculated concentrations of uninoculated BMG
Y medium show a greater than threefold higher NOEC levels of Cu and greater than 10-fold higher NOEC levels of Zn (Tables 3 and 4). In addition, trace metals may be present in media, buffer, and excipient ingredients, especially complex (undefined) nutrients and composite blends such as yeast nitrogen base. For an example dry ingredient with a measured trace metal value of 100 ppm, a 10-g/L solution of this component results in 1 ppm (1 mg/L) of trace metals in solution (Table 5), an amount similar in magnitude to the trace elements expressly added to the fermentation medium. Furthermore, trace metal composition in common raw materials can vary considerably by vendor and grade, even for inorganic salts and sugars.
Table 5: Reported values of trace elements in common media, buffer and formulation ingredients.
High extracellular concentrations of media components, such as trace metals, often inhibit cell growth. In one E. coli study, growth was inhibited at levels >8.7 g/L Mg, >0.8 g/L Mo, >44 mg/L B, >4.2 mg/L Cu, >68 mg/L Mn, >0.5 mg/L Co, >38 mg/L Zn, and >1.15 g/L Fe (4). In another E. coli study, levels of 1 mm/L Fe+3 and 0.5 mm/L Fe+2 reduced growth, and 1 mm/L Fe+2 inhibited growth (5). Compared with the NOEC/LC50 limits in Table 3, however, growth inhibition of the culture may not be evident before waste stream concentrations exceed acceptable limits. The reliability of cultivation performance as a toxicity indicator depends on the specific culture and process as well as the calculation and other desired safety factors assumed when evaluating potential discharge toxicity. For comparison purposes, process concentrations are directly compared with toxic concentrations to provide a worst-case assessment without discharge safety factors that are commonly used (Table 2).
Interestingly, there is a certain desirable level of trace elements for industrial wastewater treatment plants. Trace metals (e.g., Co, Ni, Fe, Cu, Zn, Mn) are rarely if ever added to ensure adequate micronutrient concentrations. Chemical speciation affects trace metal bioavailability because relative uptake rates differ for different chemical species and relative concentrations of chemical species differ based on exposure conditions (6). For example, free Co concentration has been shown to correlate with Co toxicity to methylotrophic methanogenesis in anaerobic granular sludge (7). Consequently, the desirability of specific trace element discharges may vary according to municipal discharge location.
The Department of Environmental Protection (DEP) in at least one state is considering regulating total dissolved solids (TDS) in new or expanded discharges to surface water and publicly owned treatment works (POTWs) to achieve tighter drinking-water quality standards (8). High TDS discharges are those >2,000 mg/L, and proposed regulations limit them to 8). Dissolved solids can be introduced directly from inorganic components (such as potassium phosphate) or indirectly from complex media ingredients (Table 5).
Table 6 shows dissolved solids levels (Mg, K, Na, and Ca) for supernatant from a Pichia fermentation. Extracellular levels of Mg consistently are about 5–10× below the 500-mg/L target level and decrease further ~5- to 15-fold during the fermentation. Ca exhibits similar behavior, starting low and generally decreasing (even when increases occurred, levels remained below the target). K patterns are notably different, starting at up to 15-fold above the target, in the best case decreasing to 0.85 g/L and in the worst case increasing to 4.1 g/L potential due to midcycle additions. Na exhibits another dramatically different behavior, typically starting at about 0.25–0.8 g/L, but at its worst case increasing well above the target to 2.9 g/L (potentially as a result of NaI present in the salt feeding solution). Measured levels for chlorides and sulfates are not available to compare with targets; however, these levels could be expected to increase because trace elements present in salt feeding solutions often possess chloride or sulfate anions (1). Consequently, the impact of process intensification on accumulated extracellular TDS levels also should be considered when examining nutrient-feeding compositions.
Table 6: Dissolved soft elements assayed in Pichia broth (Merck data).
Downstream Process Operations: Components of higher volume downstream processing and formulation buffers should be examined as suggested for fermentation-media components. Many buffers comprise relatively dilute inorganic or organic salts (6–50 mM sodium phosphate, sodium acetate, or Tris HCl) or dilute bases (50 mM sodium hydroxide). High-salt buffers are commonly used for ion-exchange (1–5 M NaCl) and hydrophobic-interaction chromatography (2 M ammonium sulfate), potentially affecting TDS discharge levels if used in large volume. Packed column sanitization or storage buffers may contain solvents (18% ethanol). Formulation buffers generally are low in volume and contain salt (25 mM sodium phosphate), amino acids (25 mM arganine), or sucrose (up to 5 wt%). Chelators, such as 1 mM ethylenediaminetetraacetic acid (EDTA) present in some buffers, potentially interfere with trace metal bioavailability in toxicity tests, reducing levels below LC50 and NOEC before waste-stream dilution and subsequent metal release in the environment.
Denaturants such as 7.5 M urea solutions are used to solubilize inclusion bodies (9). Cyanate concentrations can reach 3–4 mM over 14 days at ambient temperature in aqueous buffered 8 M urea solutions and up to 20 mM at equilibrium above pH 6.0 (10). Cyanate is substantially less toxic than cyanide (which can have discharge limits as low as Daphnia magna of 18 mg/L or 0.4 mM (11), suggesting that large-volume discharges of aged urea solutions should be carefully evaluated. Alternatives to chemical denaturation include 0.5% Sarkosyl surfactant solutions to solubilize inclusion bodies. Refolding is then achieved with 0.08 mM copper sulfate in 14 mM Tris HCl buffer, about an order of magnitude less copper than is contained in typical initial fermentation medium.
Very high and low pH streams used in bioprocesses often can be appropriately neutralized by pH control systems at site wastewater collection points. These streams include ammonium hydroxide, sodium/potassium hydroxide, phosphoric acid, sulfuric acid (usually industrial fermentations) for pH adjustments during cultivation as well as for cleaning of bioprocess equipment (12). They also include acidic concentrated sugar solutions (e.g., 200–300 g/L glucose, pH 3.0–3.5) for fed-batch carbon additions (13). Low and high pH excursions impair wastewater plants by dispersing flocs or inhibiting microbial populations (14). Furthermore, a combination of high pH and fats or fatty acids (particularly antifoams, detergents, and surfactants) can cause foaming in wastewater systems (e.g., aeration tanks) (14,15).
Other biopharmaceutical waste streams arise from sanitization solutions (usually diluted bleach) and wipe-down solutions (often quaternary disinfectants). These can be potential sources of chlorides but generally are diluted to a few percent and typically used at low volumes for wipe-downs. Highe
r volumes associated with process equipment sanitization are limited because several facilities are moving from chemical to steam sanitization procedures because of regulatory preferences. This shift puts additional pressure on companies to reduce steam energy consumption to avoid offsetting the benefits of reduced rinsewater from chemical sanitization.
Special additives such as inhibitors are often added to harvested broth to stabilize protein quality before isolation. The peptide protease inhibitors pepstatin A and chymostatin are added to Pichia broth, achieving final concentrations of 0.05–1 mg/L. An O-glycosylation inhibitor, protein O-mannosyl transferase inhibitor (PMT), is also added to Pichia broth, achieving a final concentration of ~15 mg/L. None of them show aquatic toxicity when tested at projected discharge broth concentrations.
Sewering of high–biological-oxygen-demand (BOD) broth can choke wastewater systems designed to treat wastewater of BOD 250 mg/L. Reported measured BODs for secondary metabolite broths range from 15,000 to 35,000 g/L (14). Based on relative cell mass concentrations, BODs for high–cell-density bacterial and yeast broths are expected to be similar or perhaps higher, suggesting prospective examination of sewering of high volumes of high–cell-mass broth. BODs for cell culture broths are likely to be at least 10-fold lower, creating less strain on the wastewater system.
Utility Waste Streams
Low dilution of wastewater also is problematic because large volumes of BOD 30-mg/L wastewater sent to a system designed to treat BOD 250-mg/L wastewater disrupt treatment by over dilution. In biopharmaceutical facilities, large water discharges are caused by
water-for-injection (WFI) and purified-water system flushing to reduce bioburden (typically ~3 min/day/use point) generating 5,000–15,000 L daily for a single system of 30 use points ranging from 1 to 1.5 in)
rejection of water from reverse osmosis water purification systems
extensive rinsing and flushing to achieve clean-in-place (CIP) acceptance limits.
In addition to treatment system disruption, large water discharges related to processing simply waste water. High water volumes used during biopharmaceutical production can be recovered by blending rejected water into incoming city water or using it as make-up water for chilled or tower water systems (if it can be appropriately segregated). Wastewater generation itself also can be reduced. When shorter water systems use point flushing times or frequencies are evaluated, they often do not adversely affect water quality but require extensive revalidation testing to implement. Procedurally specified flush times may already have overage incorporated (safety factors based on the validated testing times), so users can be trained to target the standard operating procedure (SOP) time exactly. Automated flush valves with shut-off timers also can help reduce wasted water, although their implementation is less frequent because of higher installation and maintenance expense. Extra rinsing or flushing during CIP procedures can be reduced by removing equipment from service to perform modifications to reduce excessive hold-up volumes and deadlegs from inadequate tube or pipe lengths or altering vessel designs to improve cleanability (16). Volumes of low-quality water used for lubrication or condensate cooling primarily in older facilities can be reduced by increasing condensate quenching temperatures to 60 °C (generally the highest value considered safe for equipment and people) and installing flowmeters and accurate valves to regulate pump seal and quench flowrates (which if unregulated can amount to as much as 2 L/min per site).
WFI costs can be a surprisingly large portion of biopharmaceutical production costs (17), generating another driver for water use reduction. Specifically, 140,000 L of buffer or cleaning solutions are required to isolate a 20,000-L fermentation (17), a sevenfold volume ratio. Buffer consumption for a protein A chromatography step is substantially reduced by lowering column volumes (CVs) (e.g., from five to three CVs for equilibration and from three to two CVs for wash steps) by 4,480 L for a 140-L column using eight cycles to purify one cell culture batch of titer of ~5 g/L (18). Higher resin loading reduces buffer and thus WFI consumption (19).
In-line buffer dilution reduces the sizes of nondisposable buffer holding tank about 10-fold (from 25,000 L to 2,500 L), which in turn reduces CIP, steam-in-place (SIP), and WFI consumption as well as physical transport of solutions within or to a facility from a vendor (18,20).Optimizing control of buffer distribution line switching is critical to minimize the line pressure disturbances that potentially alter chemical composition and generate large quantities of off-specification waste buffer (18,19,20,21).
Electricity
HVAC: The number and size of HVAC units, dictated by a biopharmaceutical facility design, can be minimized by reducing classified operating space and using the least stringent permitted levels of classification for a retained space. The large number of dedicated HVAC units in a facility is expected to remain running constantly to preserve desired cleanliness and pressurizations even when not in use. Because regulatory airflow rate standards cannot be changed readily, improvements can instead be gained by using more energy-efficient filters, air handlers, and lighting (22,23). HVAC “setbacks” in operating areas not heavily operating (because people and equipment are not frequently moving in and out) have particular impact in bioprocess facilities operating under capacity. These setbacks might be as minimal as decreasing air-handler temperatures by 3–5 °F (10% improvement) or reducing air changes from 20 to 15 for a Class 100,000 area. Such changes need to balance the additional monitoring level required to ensure the proper air quality is available when processing resumes.
Controlled Temperature Units (CTUs): Refrigerants are by far the largest burden on greenhouse gasses. In addition to large numbers of dedicated HVAC units, even larger numbers of primary and backup CTUs exist in biopharmaceutical facilities, primarily in the form of cold and frozen storage. Operators can shut off these units when they are not in use and ensure CTUs are mostly full rather than mostly empty to reduce the number of units and to minimize cycling to maintain target temperatures when doors are opened. A reduction in the amount of inventory, particularly inventory requiring controlled temperature storage such as intermediate hold streams, would further reduce CTU demands.
Air Emissions
In the United States, hazardous air pollutants (HAPs) are regulated by maximum available control technology (MACT). This standard affects methanol (MeOH) addition in methylotropic Pichia processes but excludes ethanol by-product production in Saccharomyces bioprocesses. Often MeOH emissions are limited because of subsurface addition lines and low extracellular levels required for high productivity. However, as fermentation scale increases >2,000–5,000 L, care must be taken to calculate emissions for facilities with site air-emission permit limits, particularly if a MeOH bolus of 0.5–1 vol% is used to start induction.
Entrained particles (in the form of cells) are not usually problematic for biopharmaceutical processes because of the use of 0.2-µm vent filters on bioreactor exhaust lines. If no dust collector is present, then nuisance dust emissions during charging of raw materials at t
he large scale may reach levels of concern. Often, large-scale commodity nonbiopharmaceutical fermentations use liquid raw materials to prevent such dust.
Only one example is evident in which process development is applied to reduce carbon dioxide, a greenhouse gas. Synthetic media are used to optimize very large–scale penicillin and cephalosporin production fermentations with the added benefit of reducing greenhouse gas emissions by 26% (24). Carbon dioxide levels in fermenter off-gas streams can range from one to 10 vol%, depending on the process and the process phase. For a Pichia fermentation with a peak oxygen update rate (OUR) of 150 mmol/L-h and a respiratory quotient (RQ) of 0.6, peak carbon evolution rates (CER) are 90 mmol/L-h (1). This translates into 2,100 mol carbon emitted per 1,000 L/day or 100 kg (220 lbs) carbon dioxide emitted per 1,000 L/day. This amount is significant but not substantial — it is about the same as driving 500 miles in a 2008 Toyota Prius (www.terrapass.com/carbon-footprint-calculator).
Energy use by employees commuting to and from a biopharmaceutical facility is a dominant influence on air emissions. Consequently, labor-saving technologies such as single-use equipment offer a collateral emission reduction benefit (25,26). When burned, one gallon of gasoline creates 20 lbs of carbon dioxide, or ~10% of the fermentation level described above. Thus, staffing requirements are an important consideration when evaluating disposable or fixed process equipment options as well as for overall process design. Of course, substantial telecommuting (e.g., up to three days/week) often is impractical for most employees working directly on bioprocesses. It is potentially possible for some desk-based support staff, with whom it has been demonstrated to increase productivity by 10–20% and raise job satisfaction (27).
Powder formulations for basal and feed media and buffers, save energy because transport and storage are more convenient (16). Preparing solutions on site reduces transportation to the site. Furthermore, using concentrated buffers reduces transport and storage within a site (18,20). Reconstitution or dilution methods are important to evaluate for ensuring that manpower, along with cleaning fixed equipment or disposable usage, is minimized as well.
The Path Forward for Biopharmaceutical Processes
Current facility design and equipment selection for approved biopharmaceutical products are already largely fixed. Because of license restrictions, operations are likely to be altered only if validation requirements are relatively low and subsequent control procedures are relatively simple. Consequently, proposed changes to reduce the environmental footprint of existing biopharmaceutical processes also must be evaluated for their associated quality risk. In-line preparation of process solutions, adjustment of intermediate composition, and removal of process stops (hold points) are all examples of changes that improve fixed and sometimes variable costs (28) as well as environmental footprints.
Process intensification not only improves productivity but also clearly results in greener facilities with reduced carbon dioxide footprints (29) and reduced cost of goods. Volumetric productivities of recombinant proteins are improved by better cell strains, raising cell concentrations, or optimizing feeding strategies (30,31). For example, bioreactor sizes have been reduced from 20 kL to 4 kL (32) or even 2 kL (29) as a result of higher antibody titers of at least 2–4 g/L (32) and high cell density fed-batch cultivations. Equipment size, number of units, and process volumes can be minimized particularly for upstream processes (16), resulting in smaller facility sizes (29,30) or larger facilities running under capacity. Smaller bioreactors have smaller harvest volumes, which also may permit use of ready-made media and buffers, thereby eliminating or reducing media and buffer preparation suite sizes (29). In addition, smaller bioreactors can lead to single-floor production facilities (and thus less structural steel) because smaller vessels do not protrude through multiple levels (29).
Purification operations are the typical processing bottleneck (12) with as many as three to five purification batches required for each harvested bioreactor. Titer increases lead to a linear increase in downstream equipment sizes and capacity, but this linear increase is not sustainable for very high titers. New chromatography resin technology, such as twofold higher dynamic binding capacity resins, is applied to maintain column sizes and thus proportionally sized buffer volumes constant (18). Buffer use, often another facility bottleneck, prompts process development efforts and unit operation selection to reduce buffer consumption (18). New combined cation- and anion-exchange membrane chromatography technology decreases buffer volume requirements (and buffer storage requirements) by as much as 95% because hold-up volumes are lower and regeneration buffers are not required (16,33). Other technologies that increase surface-to-volume ratios serve to decrease equipment linear dimensions and thus result in smaller equipment sizes (34).
Recent focus has shifted from product capture to contaminant removal, which has significant implications (12). For example, extra column flushes and diafiltration volumes often are added to increase process robustness for removing host cell impurities. A focus on lower cost of goods drives process development to reduce the number of process steps and eliminate unnecessary steps using improved process designs or new technology. Examples include reducing chromatography steps from three to two and omitting buffer exchange steps by designing subsequent steps to directly accept the eluate of a prior step (35). Eluates of a previous chromatography step are often diluted 1.7-fold to lower their conductivity for optimal performance in a subsequent chromatography step. This dilution necessitates large tank volumes and might be avoided by changing column order or elution conditions (18).
Moving forward, evaluation of biopharmaceutical processes early in the development phase is critical to reducing resource use and achieving target environmental measures. Prospective environmental footprints can be developed for various discharge sources, along with proposed mitigations and their drawbacks (Table 7). Proposed technology improvements also can be evaluated for their footprint impact, with opposing and supporting effects identified for different footprint areas (Table 8). Formal tracking of environmental measures (E-factors or process mass index) quantitatively demonstrates progress in environmental footprint reduction as process development advances for the same product or as platform development evolves for successive products. Such tracking can surface conflicting process design alternatives. Existing milestone reviews by internal governance committees can be leveraged and linkage to quality by design (QbD), and process analytical technology (PAT) initiatives can be explored. The evaluation framework then can be transferred to internal or contract manufacturing organizations to develop and monitor processes for future sustainability.
Table 7: Typical sources of excess water discharge in a bioph
armaceutical facility, along with potential mitigation ideas and associated drawbacksTable 8: Typical sources of excess water discharge in a biopharmaceutical facility, along with potential mitigation ideas and associated drawbacks.
About the Author
Author Details
Beth Junker is senior scientific director at Merck and Company, RY804-130, PO Box 2000, Rahway, NJ 07065; [email protected].
1.) Potgieter, T. 2009. Production of Monoclonal Antibodies By Glycoengineered Pichia pastoris. J Biotechnol. 139:318-325.
2.) Verslycke, T. 2003. The Toxicity of Metal Mixtures to the Estuarine Mysid Neomysis integer (Crustacea: Mysidacea) under Changing Salinity. Aquatic Toxicol. 64:307-315.
3.) Wang, DIC. 1979. Fermentation and Enzyme Technology, John Wiley and Sons, New York:94.
4.) Riesenberg, D. 1991. High-Cell-Density Cultivation of Escherichia coli. Curr. Opin Biotechnol. 2:380-384.
5.) Kalantari, N, and S. Ghaffari. 2008. Evaluation of Toxicity of Heavy Metals for Escherichia coli Growth. Iran. J. Environ. Health. Sci. Eng. 5:173-178.
6.) Environmental Protection Agency 2007. Aquatic Life Ambient Freshwater Quality Criteria: Copper.
7.) Bartacek, J. 2008. Cobalt Toxicity in Anaerobic Granular Sludge: Influence of Chemical Speciation. J. Ind. Microbiol. Biotechnol. 35:1465-1474.
8.) Marcellus Shale Wastewater Partnership Permitting Strategy for High Total Solids (TDS) Wastewater Discharges.
9.) Pizarro, SA. 2009. Biomanufacturing Process Analytical Technology (PAT) Application for Downstream Processing: Using Dissolved Oxygen As an Indicator of Product Quality for a Protein Refolding Reaction. Biotechnol. Bioeng. 104:340-351.
10.) Lin, M-F. 2004. Ion Chromatographic Quantification of Cyanate in Urea Solutions: Estimation of the Efficiency of Cyanate Scavengers for Use in Recombinant Protein Manufacturing. J. Chromatogr. B 803:353-362.
11.) Dauchy, JW, WT Waller, and MD Piwoni. 1980. Acute Toxicity of Cyanate to Daphnia magna. Bull. Environ. Contam Toxicol. 25:194-196.
12.) Hoffman, M. 2009. Easing the Bottleneck. Pharm. Technol. 33:44-50.
13.) Moyer, AJ, EJ Umberger, and JJ Stubbs. 1940. Fermentation of Concentrated Solutions of Glucose to Gluconic Acid: Improved Process. Indus. Eng. Chem. 32:1379-1383.
14.) Howe, RHL 1962. Complete Biological Treatment of Antibiotic Production Wastes. Biotechnol. Bioeng. 4:161-170.
15.) Sustarsic, M. 2009. Wastewater Treatment: Understanding the Activated Sludge Process. Chem. Eng. Progress 105:26-29.
16.) Alahari, A. 2009. Implementation of Cost-Reduction Strategies for HuMAb Manufacturing Processes. BioProcess Intl. 7:S48-S54.
17.) Farid, SS 2009. Economic Drivers and Trade-offs in Antibody Purification Processes. BioPharm Intl. 7:S37-S42.
18.) Trexler-Schmidt, M. 2009. Purification Strategies to Process 5 g/L Titers of Monoclonal Antibodies. BioPharm Intl. supplement.:8-15.
19.) Ho, SV. 2010. Environmental Considerations in Biologics. Green Chem. 12:755-766.
20.) Matthews, T. 2009. An Integrated Approach to Buffer Dilution and Storage. Pharm. Manufacturing 8:36-39.
21.) Patterson, BJ 2009. A Closer Look at Automated In-Line Dilution. BioPharm Intl. 22:52-65.
22.) Meyerhoff, W. 2009. Good Housekeeping: The Art of Contamination Controls. Life Science Leader 1:14-15.
23.) Elvidge, S. 2009. The “Greening” of Cleanrooms. Life Science Leader 1:22-23.
24.) Thomas, P. 2009. Big Steps, Shrinking Footprint at Novartis. Pharma. Manufacturing 8:11.
25.) Sinclair, A. 2008. The Environmental Impact of Disposable Technologies. BioPharm Intl. Supplement:4-15.
26.) Leveen, L. 2009. Single-Use Technology and the Carbon and Water Footprints of Biopharm Manufacturing. Amer. Pharma. Review 12:72-78.
27.) Nidumolu, R, CK Prahalad, and MR Rangaswami. 2009.Why Sustainability Is Now the Key Driver of InnovationHavard Business Review:57-64.
28.) Jagschies, G. 2008. State of Bioproduction, Cheaper Ways or Smarter Solutions?. BioProcess Intl. 6:96.
29.) Guidager, N. 2009. Next-Generation Facilities for Monoclonal Antibody Production. Pharma. Technol. 33:S68-S73.
30.) Panda, AK 2003. Bioprocessing of Therapeutic Proteins from the Inclusion Bodies of Escherichia coli. Adv. Biochem. Eng. Biotechnol. 85:43-93.
31.) Hacker, DL, M DeJesus, and FM Wurm. 2009. 25 Years of Recombinant Proteins from Reactor-Grown Cells: Where Do We Go from Here?. Biotechnol. Advances 27:1023-1027.
32.) Anicetti, V. 2009. Biopharmaceutical Processes: A Glance into the 21st Century. BioProcess Intl 7:S4-S11.
33.) Zhou, J. 2008. Implementation of Advanced Technologies in Commercial Monoclonal Antibody Production. Biotech. J. 3:1185-1200.
34.) Jenck, JF, F Agterberg, and MJ Droescher. 2004. Projects and Processes for a Sustainable Chemical Industry: A Review of Achievements and Prospects. Green Chem. 6:544-556.
35.) Farid, SS 2009. Economic Drivers and Trade-Offs in Antibody Purification Processes. BioPharm Intl. 7:S37-S42.
36.) Gurramkonda, C. 2009. Simple High-Cell Density Fed-Batch Technique for High-Level Recombinant Protein Production with Pichia pastoris: Application to Intracellular Production of Hepatitis B Surface Antigen. Microbial Cell Factories 8:13-20.
37.) Hang, H. 2009. A Simple Fermentation Strategy for High-Level Production of Recombinant Phytase by Pichia pastoris Using Glucose as the Growth Substrate. Enzyme Microbial Technol. 44:185-188.
38.) George, H, and W. Herber. 1998. Culture Medium for Saccharomyces cerevisiae.
39.) Wegner, EH 1986.. Biochemical Conversions by Yeast Fermentation at High Cell Densities US Patent 4,617,274.
You May Also Like