Intellectual Property and COVID-19
October 28, 2021
The COVID-19 pandemic is the greatest global health threat caused by a virus since the influenza pandemic of 1918 (and, before that, innumerable smallpox outbreaks throughout history). So far, the number of infections and deaths has not reached levels seen during the “Spanish flu” pandemic. However, travel, global trade, and modern factors such as misinformation on social media have increased infection rates and risks of infection. As of 15 July 2021, the World Health Organization (WHO) had reported 188,655,968 confirmed cases and 4,067,517 deaths worldwide (1).
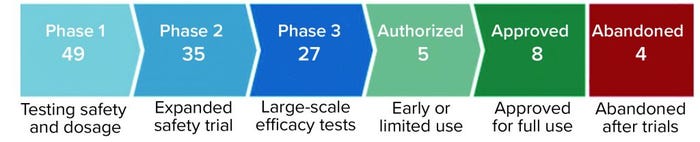
Figure 1: Coronavirus vaccine tracker (Financial Times, 26 April 2021)
Throughout most of the world — particularly in the United States, Europe, and Japan — patents provide incentives for the development of vaccines, small-molecule and biologic antiviral drugs, and other drug products. Patents protect a developer’s investments of time and resources. The rapid development of COVID-19 vaccines is an example of the benefits that incentives patents provide for protecting these investments. The speed with which anti-COVID vaccines have been developed is remarkable. By comparison, the most recent novel major vaccine against an infectious viral disease (mumps) took about three years to develop in the mid- to late-1960s. The difference is in large part because of advances in technology supported by incentives patents.
The basis for the BioNTech–Pfizer and Moderna vaccines is mRNA technology, which was developed after many years of research and supported by the prospect of patents. Similar investments in basic research and the applications of those studies to human health are important for other COVID vaccines now being administered. These include vaccines provided by Oxford–AstraZeneca, Novavax, Johnson & Johnson, and several others from China, Russia, and India, which are being used to vaccinate individuals worldwide, and many more inoculates are in development (Figure 1). To date, five billion vaccine doses have been administered worldwide (1).
Different approved COVID treatments and potential treatments have been and continue to be developed in the short time since the novel coronavirus was first recognized as a significant human pathogen. Two dozen therapies based on monoclonal antibodies (MAbs) are progressing through clinical trials as of March 2021. One treatment, Veklury (remdesivir), has received emergency use authorization (EUA) from the US Food and Drug Administration for severe cases — despite some studies failing to show survival benefit (2). Regeneron has two MAb drugs (casirivimab and imdevimab), and Eli Lilly & Co. has one (bamlanivimab) with EUAs for administration in patients older than 12 years of age with mild to moderate COVID-19 infection.
The severity of the COVID pandemic and the concomitant demand for both treatments and vaccines have increased the need for the global patent system to respond quickly to industry needs. But some people have raised concerns that intellectual property (IP) protection for COVID-19 vaccines and therapies would inhibit their development or availability. Advocates for IP protection counter that it is vital to the development of such products. The reality is that opposition to IP protection for COVID-19 vaccines, therapies, diagnostics, and other technologies can only restrict prevention, treatment, and eradication of the disease. Below, I focus on the pros, cons, and considerations of IP protection for treatments, vaccines, diagnostics, and other technologies.
Does IP Help or Hinder Product Development?
Some critics believe that IP issues or disputes could inhibit the development or distribution of such products for COVID-19 (3). But instances of those effects are lacking and in fact contrafactual (as noted above, both treatments and vaccines have been developed rapidly in the context of patent protection availability). Other experts have addressed these purported issues by calling for companies that develop those therapies to refrain (voluntarily or under duress) from asserting IP rights.
For example, in spring 2020, several universities and companies in the high-technology sector proposed the “Open COVID Pledge,” to “make our intellectual property available free of charge for use in ending the COVID-19 pandemic and minimizing the impact of the disease” (4). Both the pledge and other efforts to prevent IP protection for COVID-19 technology failed to gain traction after rebuttal by several pharmaceutical industry executives, government leaders, and politicians who understand the cost and challenge entailed by drug development (5, 6).
For example, Jon Söderström, (managing director of Yale University’s Office of Cooperative Research) noted, “The system is working. Dozens of companies and universities are now investigating COVID-19 vaccines, and more companies are researching treatments. If we strip away intellectual property rights, the system will break down, and we’ll find ourselves farther from ending our global health crisis” (7).
Health experts in favor of IP protection for COVID-19 vaccines, treatments, tests, and technologies have argued that IP protection laws should be strengthened to provide incentives for innovation and investment in products such as vaccines (which usually are unpredictable, risky, and expensive to develop). Jan Fischer, former prime minister of the Czech Republic, stated that if a COVID-19 vaccine is produced, “robust IP laws” should be given some credit for that outcome (8). Leaders from the International Chamber of Commerce (ICC) also voiced support for strong IP protection, which they noted already protects the public from counterfeit and adulterated drugs and from stockpiling medicines in developed countries (9).
The facts have not borne out early arguments that patents would inhibit vaccine development. It has been projected that more than nine billion doses will have been produced by the end of 2021. Difficulties related to global vaccination do remain, however, including the need for 2–3 billion more doses, but the current vaccination progress does rebut the argument —really a presumption — that patents would impede vaccine development.
The focus has shifted to vaccine access and COVID treatments. India and South Africa have proposed that the World Trade Organization (WTO) suspend IP enforcement provisions of the General Agreement on Tariffs and Trade (GATT) and the Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS) for all IP related to COVID, including vaccines and treatments (10). That proposal is not limited to patents; it also includes copyright and trade secrets. The proposal has been supported by mainly academics, policy organizations, and the governments of countries that would stand to benefit and (mostly) have no significant IP that would be put at risk (11–13).
The impact of India and South Africa’s proposal, if adopted, is much more threatening than merely to the development of effective COVID vaccines and treatments. Patents are published, and disclosed technologies are dedicated to the public when their term has expired. But relaxing other IP protections will destroy the IP rather than share it.
The available evidence supports the conclusion that, even if there might be circumstances under which IP diminishes vaccine availability (of which there is no evidence to date), suspending patent protection is not ideal. That is because the rate-limiting step for COVID-19 vaccine production (at least for mRNA-based vaccines) involves proprietary machines and methods that are more likely not to be covered by patents and never will be (14). Thus, adopting the type of broad IP waiver espoused by India and South Africa (that encompasses trade secrets as well as patents) would constitute an even more dangerous attack on IP.
Trade secrets (such as those used for vaccine formulation) have turned out to be important components of IP rights related to vaccine production. That form of IP rights cannot be suspended, and disclosure destroys the secret and thus the property. Policy proposals such as the WTO (broad) IP waiver have been challenged by some of the same individuals who responded to earlier IP-limiting proposals (15–17). For example, the director-general of the International Federation of Pharmaceutical Manufacturers and Associations argues that scaling up manufacturing is the best way to inoculate the world and end the pandemic. “IP protections are not barriers to access. Waiving them wouldn’t deliver a single extra dose” (18). The European Parliament has voted not to support the waiver, but the Biden Administration through its trade representative announced to much surprise that the United States would not oppose the waiver (albeit without endorsing any specific form that it might take) (19, 20).
This “moveable feast” of policy rationales illustrates the fact that the aim of the proposed WTO IP waiver and other proposals by India, South Africa, and other countries is to escape the TRIPS requirement for recognizing and enforcing IP protections, which were imposed as part of the requirements for WTO membership. When these facts are considered, the call by those governments should be understood for what it is: an attempt to use the pandemic to achieve a goal of status quo ante (prior to the establishment of the GATT/TRIPS/WTO global trade and patent regime), which was imposed upon those countries a generation ago. The COVID pandemic provides the humanitarian excuse for a solution that isn’t truly a solution but that resonates with uninformed (albeit generally well-meaning) politicians, humanitarians, and religious and nongovernmental organizations.
What Are Potential Real Solutions?
Much bigger barriers to widespread vaccination are the manufacturing, distribution, financing, and infrastructure required to support that effort (21–22). For example, the European Commission imposed a temporary export mechanism for vaccines, which first was used by Italy to block export of 250,000 doses of the AstraZeneca–Oxford COVID-19 vaccine to Australia. In turn, that caused delays in exporting vaccines, noncommercial clinical trial samples, and testing materials outside the European Union (23, 24).
Problems regarding the availability of drugs in countries with low- and middle-income economies need to be addressed. But solutions to such problems can be achieved without challenging the IP that made possible the vaccines that now must be produced and distributed. More productive routes to achieving those solutions would be focused better on open and resilient global supply chains, further cooperation among industry players to increase production, strengthened financing for and sharing vaccines with low- and middle- income countries, and regulatory approval of new vaccines.
Open and Resilient Global Supply Chains: The European Union is trying to achieve this approach with the Trade in Healthcare Products initiative at the WTO as part of the Ottawa Group. The objective is to speed up cross-border trade of final vaccines and intermediate products that could help scale up production.
The number of industry collaborations related to COVID-19 among biopharmaceutical companies continues to increase. Examples include Merck working with Johnson & Johnson, Novartis with Pfizer–BioNTech, Sanofi with Pfizer–BioNTech, GlaxoSmithKline with Curevac, Rovi with Moderna, and Novavax with Baxter. Partnerships among pharmaceutical companies can increase the number of available vaccine doses.
Several voluntary licenses have been concluded between vaccine producers and producers with the right facilities and quality levels to produce in license. For example, Astra Zeneca has signed more than 20 such agreements in 15 countries.
Strengthened Financing: Groups such as Gavi (which funds vaccines for low-income countries), the World Health Organization (WHO), and the Coalition for Epidemic Preparedness Innovations (CEPI, which finances and coordinates vaccines for outbreaks) can be involved in concerted efforts to obtain vaccine supplies for worldwide use. Western governments with “excess” vaccine reserves can use the auspices of those groups to send doses to low- and middle-income countries and economies and even some wealthier countries with the economic capacity to defray some or all costs. On 1 May 2021, the Biden administration announced that it would make available “excess” vaccines to countries in need, amounting to 60 million doses. The European Union had exported 35 million vaccines as of March 2021.
Expedited regulatory approval (e.g., under FDA’s EUA in the United States) is needed for vaccines that are in phase 3 clinical trials, if they meet regulatory criteria. That would make other vaccines available for global vaccination efforts. Currently eight vaccines have been approved worldwide, five are authorized for early or limited use, and 27 are in phase 3 (Figure 1).
The motivations for such efforts do not need to rely exclusively on altruism. The SARS-CoV-2 virus already has mutated, and further mutations can arise with variants of unknown resistance to current vaccines. Vaccines, particularly those based on mRNA, might not be effective against such variants (25). Thus, it is in everyone’s interest to extend vaccination globally to reduce the probability of such variants arising — regardless of how daunting that work might be.
IP Protection Is Needed
Efforts being applied globally to develop vaccines, treatments, and improved tests and technologies in response to COVID-19 have been impressive. We can hope that such work will prove to be successful. IP protection has a role to play in such efforts. Past experience and recent developments suggest that protecting IP for vaccines, therapies, and technologies to fight COVID-19 will have a positive impact to advance the cause of eradicating, treating, and preventing this disease.
References
1 WHO Coronavirus (COVID-19) Dashboard; https://covid19.who.int.
2 McNamara D. Large Remdesivir Study Finds No COVID-19 Survival Benefit. Medscape 15 July 2021; https://www.medscape.com/viewarticle/954888.
3 Koons C. The Vaccine Scramble Is Also a Scramble for Patents: Intellectual Property Disputes Throughout the Drug Supply Chain Could Hold Back a COVID-19 Shot. Bloomberg Bus. Week, 12 August 2020; https://www.bloomberg.com/features/2020-covid-vaccine-patent-price.
4 Open COVID Pledge; https://opencovidpledge.org.
5 Silverman E. Pharma Leaders Shoot Down WHO Voluntary Pool for Patent Rights on Covid-19 Products. STAT, 28 May 2020; https://www.statnews.com/pharmalot/2020/05/28/who-voluntary-pool-patents-pfizer.
6 Gurry F. Intellectual Property, Innovation, Access, and COVID-19. WIPO Magazine, June 2020; https://www.wipo.int/wipo_magazine/en/2020/02/article_0002.html.
7 Söderström J. Intellectual Property Makes Sure Drug Makers Deliver. Hartford Courant, 28 June 2020; https://www.courant.com/opinion/op-ed/hc-op-soderstrom-intellectual-property-drug-prices-0628-20200628-thwjarlckvezvgw3ktqkzqdpbm-story.html.
8 Fischer J. When Researchers Discover A COVID-19 Cure, Credit Should Go to Robust IP Laws, Says Former Czech PM. Int. Bus. Times, 15 Aug. 2020; https://www.ibtimes.com/when-researchers-discover-covid-19-cure-credit-should-go-robust-ip-laws-say-former-3028803.
9 How Intellectual Property Can Strengthen Our Response to Climate Change and COVID-19. International Chamber of Commerce: Paris, France, 24 April 2020; https://iccwbo.org/media-wall/news-speeches/how-intellectual-property-can-strengthen-our-response-to-climate-change-and-covid-19.
10 Waiver from Certain Provisions of the TRIPS Agreement for the Prevention, Containment, and Treatment of COVID-19. Communication IP/C/W/669. World Trade Organization, Geneva, Switzerland, 2 October 2020; https://docs.wto.org/dol2fe/Pages/SS/directdoc.aspx?filename=q:/IP/C/W669.pdf&Open=True.
11 Rutchman AS. The Intellectual Property of COVID-19, Forthcoming in Outsmarting Pandemics. Kirley E, Porter D, Eds. Saint Louis University School of Law: Saint Louis, MO, 2021; https://scholarship.law.slu.edu/faculty/533.
12 Lindsey B. Why Intellectual Property and Pandemics Do No Mix. Brookings Institute: Washington, DC, 3 June 2021; https://www.brookings.edu/blog/up-front/2021/06/03/why-intellectual-property-and-pandemics-dont-mix.
13 Over 120 Countries Back IP Rights Waiver on Covid-19 Vaccines. Pharm. Technol. 7 May 2021; https://www.pharmaceutical-technology.com/news/ip-waiver-covid-19-vaccines.
14 Lowe D. Myths of Vaccine Manufacturing. Sci. Transl. Med., 2 February 2021; https://blogs.sciencemag.org/pipeline/archives/2021/02/02/myths-of-vaccine-manufacturing.
15 Söderström J. Patents Don’t Hamper Access to Drugs and Vaccines. Boston Herald, 26 April 2021; https://www.bostonherald.com/2021/04/26/soderstrom-patents-dont-hamper-access-to-drugs-and-vaccines.
16 Iancu A. No Evidence That Patents Slow Access to Vaccines. STAT, 13 April 2021; https://www.statnews.com/2021/04/13/no-evidence-patents-slow-vaccine-access.
17 Hutterer M. Interfering with Patent Protection Means Playing with Fire. Max-Planck-Gesellschaft: Munich, Germany,
15 March 2021; https://www.mpg.de/16579491/patent-protection-vaccines-covid-10-reto-hilty.
18 Cueni T. Waiving IP Rules Will Not Deliver More Covid Vaccines. Finan. Times
25 April 2021; https://www.ft.com/content/0902d3fe-3d37-490c-a617-fbfc49dac642.
19 Fortuna G. MEPs Vote Down Call for COVID Vaccine IP Waiver. Euractiv, 30 April 2021; https://www.euractiv.com/section/coronavirus/news/meps-vote-down-call-for-covid-vaccine-ip-waiver.
20 Noonan K. Biden Administration Supports Waiver of IP Protection for COVID-19 Vaccines. PatentDocs, 5 May 2021; https://www.patentdocs.org/2021/05/biden-administration-supports-waiver-of-ip-protection-for-covid-19-vaccines.html#comments.
21 Callaway E. The Unequal Scramble for Coronavirus Vaccines – By the Numbers. Nature 584(7822) 2020: 506–507; https://doi.org/10.1038/d41586-020-02450-x.
22 Evenett S. Export Controls on COVID-19 Vaccines: Has the EU Opened Pandora’s Box? Global Trade Alert, 31 January 2021; https://www.globaltradealert.org/reports/61.
23 Nawrat A. EU/AZ Covid-19 Vaccine Row: What is the Exit Strategy. Pharm. Technol., 4 February 2021; https://www.pharmaceutical-technology.com/features/eu-az-vaccine-row-covid-19.
24 Evenett SJ, et al. The COVID-19 Vaccine Production Club: Will Value Chains Temper Nationalism? The World Bank: Washington, DC, 5 March 2021; https://documents.worldbank.org/en/publication/documents-reports/documentdetail/244291614991534306/the-covid-19-vaccine-production-club-will-value-chains-temper-nationalism.
25 Noonan K. Do mRNA-Based COVID Vaccines Have an Achilles Heel? Patent Docs, 26 January 2021; https://www.patentdocs.org/2021/01/do-mrna-based-covid-vaccines-have-an-achilles-heel.html.
Kevin E. Noonan, PhD, is partner with McDonnell Boehnen Hulbert & Berghoff LLP; [email protected].
You May Also Like





