Evaluating Freeze–Thaw Processes in Biopharmaceutical Development - Small-Scale Study Designs
Regulations mandate that biopharmaceutical product quality be controlled throughout manufacturing, storage, transportation, and delivery to patients (1). Operations often include freezing and thawing of a bulk drug substance, dilution of that purified substance to a target concentration, filtration, filling into a selected container–closure system, additional processing (e.g., lyophilization), inspection, packaging, storage, transport, and delivery (2).
Freezing is a common processing step used to maintain stability and quality of a drug substance during development and production of biopharmaceutical products. It is generally agreed upon that freezing drug substance maximizes productivity and reduces overall production costs by decoupling bulk solution manufacture and storage steps from final product manufacture. Freezing provides flexibility and cost savings by enabling batch processing: Large volumes of an expensive biological drug substance can be frozen in batches to allow the drug product to be manufactured based on real-time commercial or clinical demands. Freezing a drug substance before drug product manufacture also reduces microbial growth risk and eliminates mechanical (agitation) stresses during transportation.
Most important, this allows for a longer shelf life. Freezing decelerates chemical degradation (because reaction kinetics depend directly on temperature) and physical degradation (immobilizing drug substance in a frozen matrix limits protein–protein interactions) (3–13). Furthermore, application of freeze–thaw processes extends to storage of process intermediates, allowing for longer hold steps between manufacturing operations. Although freezing is considered to be a conservative approach, frozen storage is one of the most efficient and reliable methods to minimize protein interactions with container–closures and extend the shelf life of biological products in solution-based formulations (13).
Drug substance (typically stored in plastic bottles or single-use plastic bags) is exposed to various interfaces on contact with container–closure systems. Likewise, drug product often is filled into multiple-component container–closure systems, such as vials or prefilled syringes with rubber stopper closures (2). Interactions with container surfaces (e.g., glass or polymers) and other constituents (e.g., silicone oil) can significantly influence drug substance and drug product stability. Such interactions may be further exacerbated with mechanical stresses such as agitation during transportation. Freezing can limit exposure of a protein to those interfaces and prevent shipping- and storage-related solution instabilities.
Overall, freezing can be leveraged to maximize protein stability throughout a supply chain. However, additional controls may be needed to limit the number of freeze–thaw cycles that occur at clinics, pharmacies, or depot sites to that supported by available stability data. Doing so ensures that product quality is not compromised.
Freeze–Thaw Related Process Challenges
Freezing and thawing a biopharmaceutical product may change the chemical and physical properties of the product solution. That in turn can stress proteins, may irreversibly denature complex macromolecular structure, and could alter their stability (7, 13). Because freeze–thaw process parameters have been identified to play a significant role in product quality through alteration of a protein’s microenvironment, gaining product- and process-specific knowledge is critical to using freeze–thaw applications (11, 14, 15).
Freezing and thawing can occur at varying rates, especially when passively performed (at an uncontrolled rate). Those rates are known to affect protein stability. No standard and agreed definition of freezing or thawing rates has yet been adopted across the industry (11, 14). Depending on the equipment used, freezing rates may be slow (<1 °C/min), intermediate (1–10 °C/min), or rapid (10–900 °C/min).
For example, fast freezing rates are achieved by immersing samples in subzero solvents or baths consisting of alcohols or liquid nitrogen (80– 900 °C/min). Thawing is also performed at various rates. Like freezing, thawing can be slow (1–5 °C/min), or intermediate (>5 °C/min). It is not always feasible to monitor temperature profiles during freezing and thawing. Therefore, in design of a systematic small-scale study, it is important to incorporate an evaluation of different freezing and thawing rates (16, 17).
Slow freezing rates can result in cryoconcentration, in which proteins and excipients form concentration gradients near the freeze front and get excluded from the ice–liquid interface (2). This can further lead to pH shifts and phase separation among those components, resulting in protein structural damage (2, 14). Exposure to concentrated solutes — due to water crystallization — also can result in a loss of a protein’s thermodynamic stability, leading to unfolding events and eventually causing aggregation.
Fast freezing rates lead to smaller ice-crystal formation, which exposes proteins to a large ice–liquid interface. Concentration and adsorption of proteins at the surface of ice crystals along the ice–liquid interface can result in their partial unfolding, increased aggregation, and decreased biological activity (17, 18). One control strategy is the use of formulation excipients that act as stabilizers (e.g., surfactants and sugar cryoprotectants) to reduce the level of protein denaturation induced upon exposure to ice-liquid interfaces (18).
Of further concern is the fact that fast freezing can entrap air in the ice. When released during thawing, the entrapped air can denature proteins as air–liquid interfaces form (19, 20). Correspondingly, slow thawing rates can result in ice recrystallization, and associated shear stress can damage proteins (21). Ideally, rapid freezing and thawing are usually preferred for protein stability (22).
Other Concerns: Published literature offers no consistent terminology or parameters to describe and execute various rates of freezing and thawing. Freeze–thaw studies in traditional stability programs use passive freezing and thawing (19). Such procedures emulate a freezing process in which a drug substance or drug product is simply placed on a shelf in a freezer storage chamber and permitted to cool and freeze at an uncontrolled rate. Temperatures can vary among different commercial freezer units used for storage, partly due to differences in their size, cooling capacity, and loads. Even within the same freezer chamber, the freezing rate experienced by a protein solution could vary depending on the freezer load and the container’s position within the chamber. Passive freezing has been shown to result in protein aggregation (18).
It has been reported that active control of freezing rates can reduce stresses that cause protein structural and functional losses. Freezing rates can be highly controlled at predetermined levels; however, such an approach requires highly specialized and expensive equipment (including freeze dryers or commercially available controlled freeze–thaw systems). Actively controlled thawing also involves specialized equipment such as programmable water baths and freeze dryers. In contrast, passive thawing can be achieved, for example, by placing a frozen container at room temperature or at 2–8 °C. Thus, the need to fully control freezing and thawing rates should be evaluated carefully. Freeze–thaw studies are the most direct approaches to evaluating the impact of freezing and thawing rates and to defining process conditions (14).
Small-Scale Designs of Freeze–Thaw Studies
Early in development, material availability is usually limited. This creates a need for reliable small-scale models for various freeze–thaw processes that mimic, as closely as possible, the processes and conditions that will occur at large scale (2). Small- scale models must be designed or selected that are predictive of potential large-scale freeze–thaw issues.
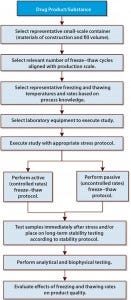
Figure 1: Small-scale study design considerations for evaluating various freezing and thawing process parameters
Figure 1 illustrates a systematic approach to designing such studies for evaluation of freezing and thawing parameters as well as protein stability following freeze–thaw stress. In general, study design starts with selection of a representative formulation and small-scale container–closure, the number of freeze–thaw cycles needed for large-scale manufacture, freezing and thawing temperatures and rates, and equipment and protocols. Study design is followed by execution of a protocol. Finally, long-term effects of freeze–thaw stress are evaluated through a stability study evaluating protein attributes under different storage conditions.
Design of small-scale studies is based on the most representative freeze–thaw conditions to be used at a manufacturing site (8). Product stability depends on selected formulation and container–closure materials. Thus, freeze–thaw evaluation first must include assessment of a product’s compatibility with the contact material of the container–closure system during freezing and thawing. Second, special attention must be paid to selecting appropriate scaled-down container– closure systems or configurations.
For laboratory-scale studies, it is important to choose an appropriate fill volume with the representative head space or surface-area-to-volume exposure. Scale-down is based on an attempt to maintain the ratio of surface area to volume similar to that at large scale. However, because of significantly different path lengths, cooling and heating rates encountered during freezing and thawing are generally much slower at large scale. Thus, process times for large-scale operations are longer than for small- scale studies, particularly with passive freezing and thawing processes. As a result, large-scale cryoconcentration becomes an important factor governing product quality. It can occur at small-scale too but is magnified by bulk freezing in which the extent of freeze concentration can be directly correlated to solution volume (3).
Moreover, it is not always feasible to monitor temperature profiles during freezing and thawing. As stated above, in designing a systematic small-scale study, it is important to evaluate different freezing and thawing rates under both active-control and passive conditions, then determine whether those rates constitute a critical process parameter (CPP) for product quality (14, 23).
Our focus herein resides primarily on the impact of freezing and thawing rates on drug substance and drug product stability in representative small-scale container– closure systems. In addition, our small-scale case studies were designed with an emphasis on adopting a robust approach to understanding interactions between formulations and process conditions. Overall, these studies systematically and comprehensively evaluate the impact of relevant processing parameters (freezing and thawing rates), formulation parameters (MAb concentration, pH, excipient levels), and container–closure systems on short- and long- term MAb storage stability. In these four case studies, we assessed the impact of varying freezing and thawing rates on the stability of
a low-concentration MAb-A drug product in glass vials
a high-concentration MAb-B drug product in glass vials and drug substance in plastic bottles
a MAb-C drug substance in single-use bags.
Here we show that understanding of a product’s robustness to freeze–thaw stress can be achieved using carefully designed scale-down models for freezing and thawing.
Case Study 1
A Controlled Freeze–Thaw Study of MAb-A at a Low Concentration (0.2 mg/mL): MAb-A was formulated as a refrigerated liquid at low concentration (0.2 mg/mL) for intravenous (IV) administration. Early clinical in-use stability studies had revealed significant adsorption onto IV bags and giving sets upon addition of the drug product to diluent in the bags due to the resulting low dosing concentration (0.002 mg/mL). To overcome significant protein loss to such binding, a surfactant (polysorbate 80, PS80) was introduced to the product formulation at a concentration of 0.1% (w/v). Although the addition of PS80 to the formulation overcame the adsorption challenges and provided protection from mechanical stress, it also accelerated chemical degradation of the MAb, in particular by oxidation. Literature shows that degradation products generated through the oxidation-induced degradation pathway lead to accelerated MAb deamidation, fragmentation, and aggregation (24). Similar behavior was observed here. Therefore, oxidation of MAb-A needed to be controlled to maintain product quality.
This oxidation challenge was identified shortly before commencement of phase 3 clinical trials. A multicomponent control strategy was devised to overcome the identified surfactant-induced chemical degradation and allow on-time start of clinical trials and submission of a licensing application. A key element of the strategy entailed changing the proposed long-term storage conditions for this drug product from refrigerated to frozen, which could sufficiently inhibit the observed chemical degradation. However, before phase 3 supplies were manufactured, it was also imperative to assess the impact of process parameters (e.g., freeze–thaw rates) on drug product quality and stability.
The experimental plan for a small- scale, freeze–thaw study of MAb-A consisted of a statistical combination of two factors at two levels each. Factor 1 was the type of stress (freeze or thaw), and factor 2 was the stress rate (slow or fast). Figure 2 shows the overall study design, which consisted of four combinations of stress and stress rates: the fast-freeze, fast-thaw, slow-freeze, and slow-thaw protocols described. All solutions were tested in glass vials (the drug product container–closure) and subjected to two freeze–thaw cycles.

Figure 2 (Case Study 1): Low- concentration MAb-A solutions in glass vials were subjected to two freeze–thaw cycles. Samples were either fast-frozen in a dry ice and acetone slurry (active fast freeze protocol) or slow-frozen at a rate of 0.1 °C/min (active slow freeze protocol) using a laboratory freeze- dryer. Frozen samples were subsequently thawed in a water bath at ambient temperature (active fast thaw protocol) or thawed at the rate of 0.1 °C/min (active slow thaw protocol) in a laboratory freeze dryer.
Active Freezing Rate | Active Thawing Rate |
Fast Freeze | Fast Thaw |
Slow Freeze | Slow Thaw |
Fast Freeze | Slow Thaw |
Slow Freeze | Fast Thaw |
As Figure 3 shows, MAb-A samples subjected to two controlled freeze–thaw cycles experienced no increase in oxidation over the initial set of samples. Deamidation (previously noted as a common degradation pathway for this MAb) also was not detected. Fluorescence intensities did not change, indicating maintenance of the overall tertiary structure and packing of the MAb when frozen and thawed at different rates. No significant changes to the aggregate or fragment profile were observed, nor was any increase of particulates in solution observed by size-exclusion chromatography (SEC) or ProteinSimple microflow imaging (MFI) analyses, respectively. No significant changes were observed in general appearance (GA), pH, or absorbance at 280 nm (A280) analyses for all samples.
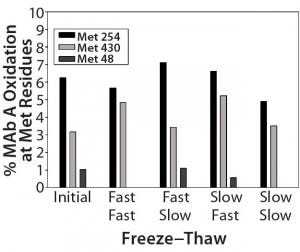
Figure 3 (Case Study 1): Summary results of %oxidation at methionine (Met) residues following two actively controlled freeze–thaw cycles for MAb-A; refer to Figure 2 for study setup and sample description.
This small-scale study design permitted rapid evaluation of the impact of extreme freezing and thawing rates on MAb-A quality. It supported implementation of freezing and thawing processes during manufacture and storage of this product in the selected container– closure system. Study results verified that control of two significant process parameters — freezing and thawing rates — would not be required during handling of this product.
Case Study 2
Evaluation of MAb-B Stability at 200 mg/mL After Short-Term Exposure to Subzero Temperatures Above Tg’: Protein concentration and container type can significantly affect MAb behavior in a frozen state. So can storage temperature in relation to the glass transition temperature (Tg′), potentially causing irreversible damage such as loss of structure through partial unfolding and adsorption to a container through exposure of hydrophobic residues (7, 11). Loss of structural integrity can directly result in aggregate formation through increased interaction of partially unfolded molecules with each other or with other species upon thawing. That phenomenon can be enhanced if the frozen storage temperature is near or above Tg′ of the formulation (25).
Stability studies revealed that MAb-B failed acceptance criteria for aggregates when frozen at –20 °C (>Tg′) for a month. A significant increase in subvisible particles also was observed. Not only were MAb-B drug substance and product both formulated at very high concentration (200 mg/mL), but also they used a buffer system with no cryoprotectant. Temperature excursions below 2 °C thus were strictly prohibited for early phase clinical supplies. However, those restrictions added significant supply-chain risk because the project progressed into later-phase clinical development. For example, according to internal shipping temperature logs (data not shown), temperature excursions outside 2–8 °C were typically in the range of –1 °C to –11 °C and tended to be on the lower end of that range. Thus, we needed to justify a more practical temperature excursion range.
A small-scale freeze–thaw procedure — together with a long- term stability study — was implemented to discharge shipment and storage excursion risks of short- term exposure to subzero temperatures >Tg′. Table 1 displays the details of the study design. MAb-B and a corresponding placebo control were filled into small-scale high-density polyethylene (HDPE) bottles and glass vials that were representative of the proposed commercial configuration. Active and placebo solutions were cooled or frozen to 0 °C, –5 °C, and –15 °C by placing filled containers in a laboratory bath containing a heat-transfer fluid. After about 72 hours of exposure to the target temperature, samples were moved to long-term storage at 2–8 °C for up to 12 months.
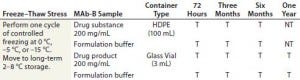
Table 1 (Case Study 2): Summary of a small-scale study designed to expose MAb-B drug substance and drug product in their representative container–closure systems to various subzero temperatures under active control
Stability of the MAb-B solutions was assessed immediately after subzero temperature treatment and again after long-term storage by general appearance, testing pH and A280, and using SEC, capillary isoelectric focusing (cIEF), ProteinSimple MFI, reverse-phase chromatography (RP-HPLC), and surface plasmon resonance (SPR) potency analysis. Structural integrity of the MAb was also determined by biophysical testing: fluorescence spectroscopy and circular dichroism. Figure 4 summarizes SEC stability results. MAb-B drug product at a concentration of 200 mg/mL exhibited no significant changes in monomer concentration or aggregate profile when stored for up to 12 months (one year) at 2–8 °C after 72-hour incubation at the three subzero temperatures.
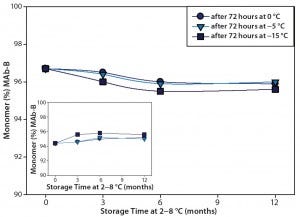
Figure 4 (Case Study 2): Summary results of %monomer and %HMW (high–molecular-weight species) by SEC for MAb-B drug product at 200 mg/mL; following one cycle of actively controlled freeze cycle (72-hour storage at 0 °C, –5 °C, or –15 °C), the product was stored at 2–8 °C for ≤12 months. Refer to Table 1 for study setup and sample description.
The results from the analytical and biophysical testing showed that MAb-B quality and stability in the selected container–closure system were not affected by short-term exposure to temperatures between –15 °C and 0 °C. These data were used to justify expansion of the excursion limits for this project, which reduced the risk of stock-outs and clinical delays.
Case Study 3
Drug Substance Robustness, Freeze–Thaw, and Long-Term Stability in Small-Scale System Configuration for a 100-mg/mL MAb-C: Commercial- scale production of drug substance is no longer limited to rigid geometric containers such as bottles and carboys. Single-use bag systems made of flexible polymeric materials offer an alternative. One such example is the Celsius FFT (flexible freeze– thaw) bag system from Sartorius Stedim Biotech. The FFT bag uses Celsius film, of which the product- contact component is ethylene vinyl acetate (EVA).
The Celsius bag was identified as the preferred container for phase 3 and commercial supply of MAb-C drug substance formulated at 100 mg/mL. Upon information review and consultation with the vendor, 5-mL Flexboy bags from Sartorius Stedim Biotech were determined to represent a suitable scale-down model of commercial sizes in the Celsius bag system.
The goal of this study was to assess robustness of the drug- substance formulation to freeze–thaw stress and subsequent storage at various conditions in the 5-mL bags. Table 2 lists the two formulation robustness factors incorporated into this study design: MAb concentration (±20% target) and pH range (±0.2 units from target). Solutions of MAb-C were aliquoted into each bag at about 40% fill-volume capacity. Samples were subjected to five freeze–thaw cycles in which the rates of freezing and thawing were passively controlled. Samples were frozen by placing them horizontally in ≤–60 °C storage chambers (passive freezing protocol) and thawed in a 2–8 °C storage chamber (passive thawing protocol), each at about 12 hours duration.

Table 2 (Case Study 3): Summary of a small-scale, passive freeze–thaw study for MAb-C using disposable 5-mL EVA bags filled at 40% volume with samples representative of formulation robustness
MAb-C stability was assessed after bags were subjected to five passive freeze–thaw cycles from ≤–60 °C to 2–8 °C and after storage at ≤–60 °C, –40 °C, –20 °C, 2–8 °C, and 25 °C for one, three, and six months. Samples were analyzed for general appearance, pH, and freezing-point osmolality, and by microfluidic chip electrophoresis (Caliper Life Sciences), SEC, cIEF, and SPR. Figure 5 displays SEC %monomer results, showing no significant change in the purity profile for MAb-C drug substance after five passive freeze– thaw cycles from ≤–60 °C to 2–8 °C followed by six-month storage at 2–8 °C regardless of sample type.
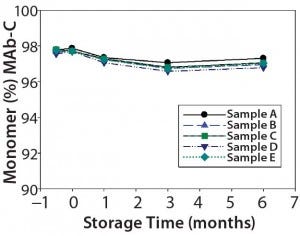
Figure 5 (Case Study 3): Summary results of %monomer by SEC for MAb-C drug substance after five cycles of passive freeze–thaw cycles from ≤–60 °C to 2–8 °C, followed by six-month storage at 2–8 °C; refer to Table 2 for study setup and sample description.
Results from this study confirm long-term stability of the MAb-C product within specified formulation robustness ranges following five passive freeze–thaw cycles. Consequently, no active freeze–thaw control was implemented at the drug- substance manufacturing site. Instead, filled Celsius bags were frozen by placing them in a standard ≤–60 °C freezer, and the substance was thawed at 2–8 °C in a cold room.
Case Study 4
Active and Passive Freeze–Thaw of MAb-C at 120 mg/mL in 30-mL Celsius Bags: Combining actively controlled freeze–thaw technology with single- use bags can greatly reduce overall cost of goods and enhance operational flexibility (26). The Celsius S3 system from Sartorius Stedim Biotech is designed to reproduce active control of large-scale freezing and thawing conditions in small-scale EVA bags (of 30 mL and 100 mL total volume) (8, 26). This study was designed to assess whether an actively controlled freezing and thawing process using the Celsius S3 system would provide an advantage over passive freezing and thawing for MAb-C. Resulting data were used to determine whether to purchase a controlled freeze–thaw system for the manufacturing site.
Figure 6 (Case Study 4): A small-scale, controlled freeze–thaw study using single-use 30-mL Celsius bags for MAb-C; solutions were aliquoted into each bag at approximately 66% fill volume. The 120-mg/mL samples were subjected to three freeze–thaw cycles in which the rates of freezing and thawing were actively controlled (table below). Samples were either frozen in liquid nitrogen (active fast freeze protocol, graph below) and then thawed in a water bath at ambient temperature (active fast thaw protocol), or frozen from 25 °C to −70 °C at a controlled rate of 0.1 °C/min (active slow freeze protocol) and thawed from −70 °C to 25 °C at a controlled rate of 0.1 °C/min (active slow thaw protocol) by using Celsius S3 system (photo below). After three freeze–thaw cycles, the bags were placed at 2–8 °C and 25 °C for long-term storage up to one, three, and six months.
Sample Set | Freezing Rate | Thawing Rate |
f | Controlled Fast | Controlled Fast |
g | Controlled Slow | Controlled Slow |
h | Controlled Fast | Controlled Slow |
i | Controlled Slow | Controlled Fast |
j | Uncontrolled | Uncontrolled |
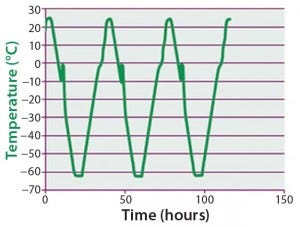
(graph) Controlled slow freeze-thaw temperature profile at 0.1 °C/min using the Celsius® S3 System
(photo) a 30-mL Celsius bag containing MAb-C BDS at 120 mg/mL after being subjected to controlled fast-freezing protocol using liquid nitrogen
Figure 6 summarizes the study design and procedure. Single-use 30-mL Celsius bags were used. Samples were subjected to three freeze–thaw cycles with different rates of freezing and thawing achieved using Celsius S3 system, a liquid nitrogen bath, and a water bath. GA, pH, freezing-point osmolality, microfluidic chip electrophoresis, SEC, cIEF, SPR, and PS80 testing were used to test MAb-C stability under stresses of extreme freeze–thaw rates followed by long-term storage (at 2–8 °C and 25 °C for up to six months) in this small-scale system configuration. As Table 3 shows, no significant changes were observed in either the size (measured using SEC) or charge (measured using cIEF) profiles of MAb-C after three freeze– thaw cycles. A similar observation was made for cycled samples stored at 2–8 °C for six months.
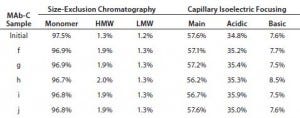
Table 3 (Case Study 4): SEC and cIEF results for MAb-C drug substance subjected to three cycles of active freezing and thawing from –70 °C to 25 °C and stored up to six months at 2–8 °C; refer to Figure 6 for study setup and sample description.
The results of this study — together with those described in case study 3 above — indicate that active control of freezing and thawing rates during manufacture was not necessary for MAb-C. Although such results may not be observed for all MAbs in development, they were not unexpected here because the formulation was specifically designed to withstand stresses that can be induced by freezing and thawing.
Demonstrating Due Diligence
Freeze–thaw processes are routinely used to improve storage and shipping stability in production and storage of biopharmaceutical solutions. However, freezing and/or thawing processes can adversely affect protein product stability and quality. For this reason, relevant studies are needed to assess formulation robustness to freeze–thaw stress and container–closure compatibility with as well as to define freezing and thawing parameters for large-scale production.
Designing freeze–thaw studies at conditions representative of stresses expected at large-scale production is a significant challenge requiring consideration of multiple factors such as container–closure material and size, fill volume, and freezing and thawing rates. Although small- and large-scale contact materials can be matched with relative ease, the significantly different path lengths make freezing and thawing rates extremely difficult to match. One approach we adopted at small scale was to show that freezing and thawing rates do not significantly influence product quality in the selected formulations. Neither freezing nor thawing rates (both significant process parameters) needed to be controlled during product handling.
In all four case studies, freeze– thaw cycles tested at small scale mimicked the product-specific system and process for potential application at large-scale production. For active control of freezing and thawing rates, we used laboratory approaches such as a freeze dryer, liquid nitrogen, dry-ice acetone slurry, and a water bath (in addition to specialized technologies such as the Celsius S3 freeze–thaw system). These studies exemplified how small-scale freeze–thaw approaches can be implemented in the biopharmaceutical industry to confirm container–closure selection, verify formulation robustness, support temperature excursion limits, set process parameters, and (most important) justify the need for either active or passive freeze–thaw procedures and associated equipment before expensive manufacturing campaigns begin. Although our intent in these studies was not to evaluate concentration effects, their results illustrate the success of such designs across a wide range of concentrations (0.2 mg/mL MAb-A in case study 1 and 200 mg/mL MAb-B in case study 2).
Understanding the product robustness to freeze–thaw stress can be achieved using carefully designed scale-down models for freezing and thawing. Also, we propose that systematic small-scale designs similar to those discussed here could be adopted as predictive, robust approaches and applied across the biopharmaceutical industry to assess the impact of freeze–thaw process on product quality and to support manufacturing, storage, and handling decisions.
References
1 Ammann C. Stability Studies Needed to Define the Handling and Transport Conditions of Sensitive Pharmaceutical or Biotechnological Products. AAPS Pharm. Sci. Tech. 12(4) 2011: 1264–1275.
2 Rathore N, Rajan RS. Current Perspectives on Stability of Protein Drug Products During Formulation, Fill and Finish Operations. Biotechnol. Prog. 24(3) 2008: 504–514.
3 Kolhe P, et al. Large-Scale Freezing of Biologics: Understanding Protein and Solute Concentration Changes in a Cryovessel — Part 2. BioPharm Int. 23(7) 2010: 40–49.
4 Kolhe P, et al. Large-Scale Freezing of Biologics: Understanding Protein and Solute Concentration Changes in a Cryovessel — Part 1. BioPharm Int. 23(6) 2010: 53–60.
5 Kolhe P, Amend E, Singh SK. Impact of Freezing on pH of Buffered Solutions and Consequences for Monoclonal Antibody Aggregation. Biotechnol. Prog. 26(3) 2010: 727–733.
6 Kolhe P, Badkar A. Protein and Solute Distribution in Drug Substance Containers During Frozen Storage and Post-Thawing: A Tool to Understand and Define Freezing- Thawing Parameters in Biotechnology Process Development. Biotechnol. Prog. 27(2) 2011: 494–504.
7 Kueltzo LA, et al. Effects of Solution Conditions, Processing Parameters, and Container Materials on Aggregation of a Monoclonal Antibody During Freeze– Thawing. J. Pharm. Sci. 97(5) 2008: 1801– 1812.
8 Le Saout X, et al. Safe Freeze–Thaw of Protein Drug Products: A QbD Approach. BioPharm Int. 23(12) 2010: 28–43.
9 Miller MA, et al. Frozen-State Storage Stability of a Monoclonal Antibody: Aggregation Is Impacted By Freezing Rate and Solute Distribution. J. Pharm. Sci. 102(4) 2013: 1194–1208.
10 Rodrigues MA, et al. Effect of Freezing Rate and Dendritic Ice Formation on Concentration Profiles of Proteins Frozen in Cylindrical Vessels. J. Pharm. Sci. 100(4) 2011: 1316–1329.
11 Singh SK, et al. Large-Scale Freezing of Biologics. BioProcess Int. 7(9) 2009: 32–44.
12 Singh SK, et al. Frozen State Storage Instability of a Monoclonal Antibody: Aggregation As a Consequence of Trehalose Crystallization and Protein Unfolding. Pharm. Res. 28(4) 2011: 873–885.
13 Webb SD, et al. Freezing Bulk-Scale Biopharmaceuticals Using Common Techniques — and the Magnitude of Freeze-Concentration. BioPharm Int. 15(5) 2002: 22–34.
14 Bhatnagar BS, Bogner RH, Pikal MJ. Protein Stability During Freezing: Separation of Stresses and Mechanisms of Protein Stabilization. Pharm. Dev. Technol. 12(5) 2007: 505–523.
15 Singh SK, et al. Large-Scale Freezing of Biologics: A Practitioner’s Review, Part 2: Practical Advice. BioProcess Int. 7(10) 2009: 34–42.
16 Kelley B. Industrialization of MAb Production Technology: The Bioprocessing Industry at a Crossroads. MAbs 1(5) 2009: 440–449.
17 Chang BS, Kendrick BS, Carpenter JF. Surface-Induced Denaturation of Proteins During Freezing and Its Inhibition By Surfactants. J. Pharm. Sci. 85(12) 1996: 1325– 1330.
18 Strambini GB, Gabellieri E. Proteins in Frozen Solutions: Evidence of Ice-Induced Partial Unfolding. Biophysical. J. 70(2I) 1996: 971–976.
19 Lashmar UT, Vanderburgh M, Little SJ. Bulk Freeze–Thawing of Macromolecules Effects of Cryoconcentration on Their Formulation and Stability. BioProcess Int. 5(6) 2007, 44–54.
20 Miller R, et al. Dynamics of Protein and Mixed Protein/Surfactant Adsorption Layers at the Water–Fluid Interface. Adv. Colloid Interface Sci. 86(1) 2000: 39–82.
21 Cao E, et al. Effect of Freezing and Thawing Rates on Denaturation of Proteins in Aqueous Solutions. Biotechnol. Bioeng. 82(6) 2003: 684–690.
22 Yan L, Zhu Z. Development of Antibody-Based Therapeutics for Oncology Indications. Drug Dev. Res. 67(9) 2006: 699– 728.
23 Heller MC, Carpenter JF, Randolph TW. Protein Formulation and Lyophilization Cycle Design: Prevention of Damage Due to Freeze-Concentration Induced Phase Separation. Biotechnol. Bioeng. 63(2) 1999: 166–174.
24 Luo Q, et al. Chemical Modifications in Therapeutic Protein Aggregates Generated Under Different Stress Conditions. J. Biol. Chem. 286(28) 2011: 25134–25144.
25 Singh SK, et al. Best Practices for the Formulation and Manufacturing of Biotech Drug Products. BioPharm Int. 22(6) 2009: 32–48.
26 Voute N et al. Disposable Technology for Controlled Freeze–thaw of Biopharmaceuticals at Manufacturing Scale. BioProcess Int. 2(9) 2004: S40–S43.
Further Reading
Elvin JC, Couston RG, van der Walle CF. Therapeutic Antibodies: Market Considerations, Disease Targets and Bioprocessing. Int. J. Pharm. 440(1) 2013: 83–98.
Reichert JM. Antibody-Based Therapeutics to Watch in 2011. MAbs 3(1) 2011: 76–99.
Karow AR, Bahrenburg S, Garidel P. Buffer Capacity of Biologics — from Buffer Salts to Buffering By Antibodies. Biotechnol. Prog. 29(2) 2013: 480–492.
Corresponding author Manasi Puri is a scientist; Sorina Morar-Mitrica is a senior scientific investigator; George Crotts is manager; and Douglas Nesta is director of biopharmaceutical product sciences at GlaxoSmithKline, 709 Swedeland Road, King of Prussia, PA 19406; 1-610-270-5900; manasi.5.puri@ gsk.com.
You May Also Like





