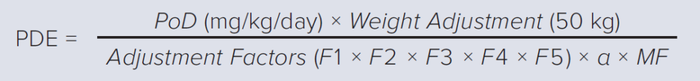Degradation of Biopharmaceuticals During Cleaning Processes: Comparing Two Different Analytical Methods for Assessment with Bispecific Antibodies
Before June 2015, pharmaceutical manufacturing of certain types of drugs required dedicated facilities. The European good manufacturing practice (GMP) guidelines state that to minimize the risk of cross-contamination, “dedicated and self-contained facilities must be available for the production of particular medicinal products, such as highly sensitizing materials (e.g., penicillin) or biological preparations (e.g., from live microorganisms). The production of certain additional products, such as certain antibiotics, certain hormones, certain cytotoxics, certain highly active drugs, and nonmedicinal products should not be conducted in the same facilities” (1).
In multiproduct manufacturing facilities that do not handle those “certain” types of drugs — which were never defined — acceptance-limit calculations for cleaning procedures followed three simple rules first articulated by Fourman and Mullen in 1993 (2):
• No more than 0.001 dose of any product will appear in the maximum daily dose of another product.
• No more than 10 ppm of any product will appear in another product.
• No quantity of residue will be visible on equipment after cleaning procedures have been performed.
In late 2014, the European Medicines Agency (EMA) adopted a risk-based approach to drug manufacturing in multiproduct facilities (3). Beginning in June 2015, multiproduct operations in shared facilities required establishment of health-based permitted daily exposure (PDE) values to ensure that residual levels from cross-contamination of drug products posed no risk to patient health. In 2018, the Pharmaceutical Inspection Cooperation Scheme (PIC/S) adopted EMA requirements and methodology for establishment of PDE values, making it obligatory among the 54 participating authorities (4). PDE values are calculated using Equation 1.
Equation 1 F1 = Factor for interspecies extrapolation based on allometric scaling from animals to F2 = Factor for interindividual variability in the human population. F3 = Factor for toxicity studies of short-term exposure. F4 = Factor for the presence of severe toxic effects. F5 = Factor for the absence of a no-observed effect level (NOEL) or NOAEL. α = Bioavailability. MF = Modifying factor (if well-supported) to address residual uncertainties not covered by the above factors. |
Cleaning processes for multiproduct facilities involve calculating the maximum allowable product carryover (MAC) — also called the maximum safe carryover (MSC) — from one product (A) to another (product B). MAC is calculated using the PDE value of product A in Equation 2:
MAC = MA × (BB, MIN ÷ DB, MAX) where MA = PDE of product A active molecule or degraded fragments, BB, MIN = minimum batch size of product B, and DB, MAX = maximum dosage of product B.
The PDE of active protein is a safety-based limit that is calculated based on toxicity data for a product. Biopharmaceutical cleaning and sterilization processes denature and degrade active pharmaceutical ingredients (APIs) into fragments that are pharmacologically inactive. If product A completely degrades during cleaning, then the residual carryover material to the next product is made up of inactive protein fragments. The PDE of such carryover used for the MAC calculation has been determined using gelatin as a reference impurity in a comparable quality approach (5).
Inactivation/degradation studies are performed at bench scale to demonstrate that a drug substance/drug product (DS/DP) has been degraded and inactivated when exposed to cleaning cycles used in manufacturing. Bench-scale studies are designed to simulate worst-case or cleaning conditions based on those encountered during full-scale manufacturing conditions.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) is widely used in the biopharmaceutical industry to demonstrate protein degradation after exposure to a cleaning process at bench scale. Inactivated study samples generated from a bench-scale study are loaded onto a gel along with control material (untreated DS/DP). The absence of molecular-weight bands corresponding to the controls demonstrates inactivation and/or degradation of the proteins in the bench-scale cleaning-process simulation.
The PDE values of highly potent biologics are significantly lower («1 µg/day) than those of most conventional small-molecule drugs. That necessitates the use of highly sensitive, validated, and robust analytical and cleaning methods to ensure that such API molecules degrade adequately during a cleaning process and that residual active API does not exceed the PDE of that protein. The minimum percentage of degradation required to achieve health-based cleaning (the PDE of a given product) is calculated by Equation 3: % Degradation = 1 — (PDE of Active Product) ÷ (650 μg) × 100%. As Table 1 shows, the minimum required percentage of degradation increases dramatically as PDE values fall, which emphasizes the need for highly sensitive, validated, and robust cleaning and analytical methods.
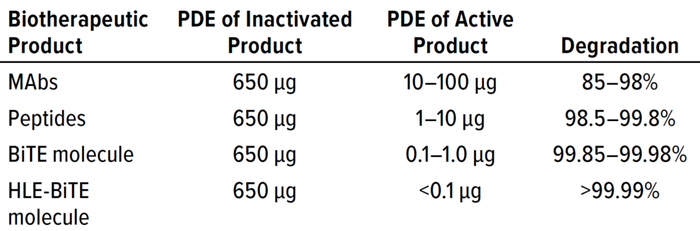
Table 1: Biotherapeutic product and degradation percentage
required during cleaning.
MAbs = monoclonal antibodies; BiTE = bispecific T-cell engager;
HLE = half-life extended.
Experiments and Data
To demonstrate >99.99% degradation (Figure 1), the difference between the concentration of a sample (assuming no degradation) and the sensitivity of an analytical method — expressed by its limit of detection and limit of quantification (LoD/LoQ) — should be adequate to demonstrate the required degradation. For DS/DPs with very low API concentrations and high potencies (low PDEs), the validated method’s limits must be correspondingly low. Below, I discuss a log-reduction approach based on SDS-PAGE and then follow by detailing the use of highly sensitive surface plasmon resonance (SPR) technology for assessing cleaning samples.

Figure 1: Sensitivity and sample concentration for required degradation.
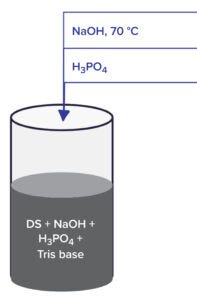
Figure 2: Bench-scale inactivation degradation study.
Bench-scale inactivation studies (Figure 2) are performed to demonstrate APIs are degraded and inactivated when exposed to the cleaning cycles used at manufacturing sites. The bench-scale cleaning conditions are intended to represent full-scale, real-world cleaning conditions that would be least conducive to inactivation of the API (worst-case conditions).
A known amount of the API is spotted onto stainless-steel coupons for a known period of dirty hold time (DHT) and kept at a known humidity. The small-scale DHT is meant to simulate a full-scale DHT, which is the time interval between the end of equipment use (drained equipment) and the start of cleaning. A reciprocal shaker bath is charged with 15 L of deionized (DI) water, then heated to 70 ± 2 °C (or a similar facility-specific temperature), and the shaker speed is set to 10 cm/sec. The coupons are placed in a sterile vial/bottle with their soiled sides facing up. An alkali wash is simulated by adding a calculated amount of alkali to reflect the pH conditions, then the container is heated to 70 ± 2 °C and placed in the shaker bath at 70 °C for a set period. Next, the alkali in the vial is titrated to a pH of 7.0 ± 0.2 with phosphoric acid (H3PO4) to stop the degradation reaction (Figure 2). Half of the degraded material is stored for analysis, and the rest is autoclaved at 121 °C for 15 minutes to simulate steam-in-place (SIP) conditions.
To simulate worst-case conditions, some steps (including rinses) are eliminated from the experimental design. To simulate a manual cleaning process, samples are prepared as above except that temperature (ambient) and a corresponding concentration of sodium hydroxide (3 N NaOH) alkali are used.
SDS-PAGE has been used across the biopharmaceutical industry for years to assess API protein degradation in cleaning processes. DS/DP test and control samples (the latter are not exposed to cleaning conditions) are used to assess the presence or degradation of protein.
Sensitivity and Degradation Percentage: As part of cleaning-sample assessment, a control sample of native protein that has not been exposed to the cleaning process is loaded onto a polyacrylamide gel to serve as a reference control sample. A sample of DS/DP after exposure to the cleaning process is loaded into another lane of the same gel. An absence in the cleaning sample of bands at the molecular weight of the native protein (the reference control sample) demonstrates degradation of the protein. This qualitative approach is helpful; to assess the degradation percentage fully, however, you need to know the sensitivity (LoD/LoQ) of your SDS-PAGE method.
Qualitative to Quantitative Transformation of Data: We loaded the lowest protein masses detectable with silver staining of two bispecific molecules at protein mass ranges of 0.5–10.0 ng. As Figure 3 shows, protein loads of ≥2.5 ng provided clearly visible bands for both molecules.
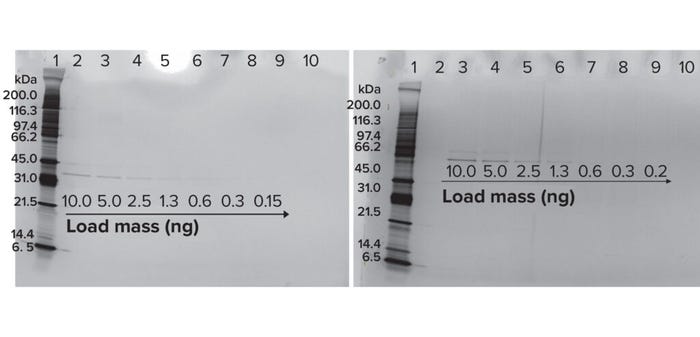
Figure 3: Sensitivity of BiTE molecules A (left) and B (right).
Degradation Percentage By SDS-PAGE: Based on 2.5 ng as the lowest concentration effectively detectable using SDS-PAGE, loading a protein mass of 50 ng demonstrates 95% degradation: (1 – (2.5 ÷ 50)) × 100% = 95%. As Table 2 shows, different cleaning sample masses loaded can demonstrate different levels of degradation. Loads >200 ng are not recommended with the silver-staining methodology because such high protein loads can cause dark smears in gel images, making it harder to visualize distinct bands at different molecular weights. For the modalities we have tested at Amgen (peptides and monoclonal antibodies), the required degradation can be met using the SDS-PAGE method with protein mass loading ≤200 ng. Based on the sensitivity and protein mass loaded in an SDS-PAGE gel, degradation >99% cannot be demonstrated. At Amgen, we use the sterility-assurance D-value (1 log reduction) approach, and studies are performed to assess the cleaning times required for 1 log (90%) degradation. As Table 3 shows, the full-scale clean-in-place (CIP) process has a 10-minute cleaning cycle, and the bench-scale study exposed the product to a 1-minute cycle (10% of the duration of the full-scale cleaning cycle) under alkaline conditions. At the end of that period, samples are neutralized with acid to stop the degradation process.
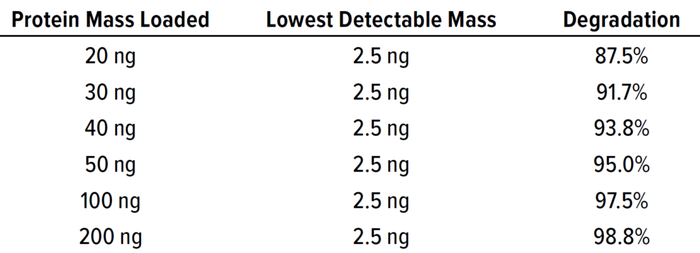
Table 2: Sodium-dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) protein mass loading, sensitivity, and degradation percentage.

Table 3: Full-scale and bench-scale caustic cycle times.
Figure 4 shows SDS-PAGE results. The cleaning-cycle samples (lane 3 in both pictures) show no bands at the molecular weights corresponding with the DS control samples (lane 2). For the gels in Figures 3 and 4, both the API cleaning and control samples were loaded at 50 ng.
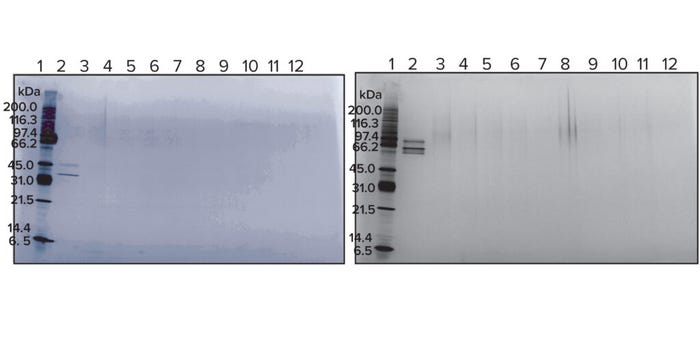
Figure 4: SDS-PAGE with cleaning samples from BiTE molecules A (left) and B (right).
SPR Methodology for Assessing Cleaning Samples: With Cytiva’s Biacore SPR technology, one interacting partner (e.g., a receptor protein) is attached to the surface of a biosensor chip, and samples containing another interaction partner (e.g., a drug ligand) pass over that surface. Binding of sample molecules to the sensor surface generates a change in resonance units (RUs) on the biosensor surface (Figure 5). Measurement of that response over time provides an association rate, and then stopping the sample flow and flushing with buffer provides a dissociation rate. From those data, you can calculate the concentration of a sample solution. In assessment of cleaning samples, a calibration curve is built with a known concentration of DS/DP, and the cleaning and calibration-curve samples are run together.
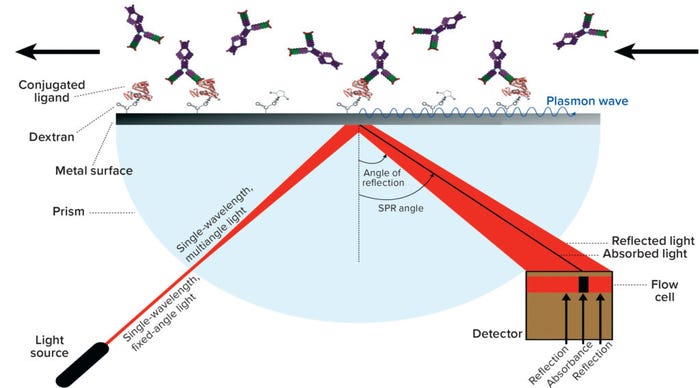
Figure 5: Principle of surface plasmon resonance (SPR) FROM SABBAN S, 1 SEPTEMBER 2011, HTTPS://COMMONS.WIKIMEDIA.ORG/W/INDEX.PHP?TITLE=SARISABBAN&ACTION=EDIT&REDLINK=1.
Sensitivity and Degradation Percentage of HLE-BiTE Molecules: Amgen’s Bispecific T-cell Engager (BiTE) technology is designed to overcome cancer-cell evasion of the human immune system by stimulating patients’ own T cells to target cancerous cells directly. BiTE molecules are engineered to bind a selected tumor-associated antigen (TSA) and CD3 found on T cells. Canonical BiTE molecules are relatively small recombinant proteins (~54 kDa), which like other biotherapeutic proteins, should degrade into small peptides and amino acids through catabolic pathways. They have a typical serum half-life of just a few hours, thus requiring continuous intravenous infusion to maintain serum concentrations at therapeutic levels.
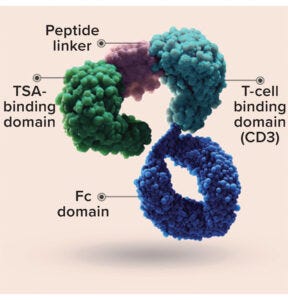
Figure 6: Half-life–extended (HLE)
bispecific T-cell engager (BiTE) molecule
(HTTPS://WWW.AMGENONCOLOGY.COM).
Amgen is designing half-life–extended (HLE) BiTE molecules containing a fragment crystallizable (Fc) domain (Figure 6). Adding the Fc domain increases the in vivo half-life of a BiTE molecule enough to support weekly dosing. Given their extreme potency, HLE-BiTE molecules require >99.99% degradation to achieve the PDE (Table 1). The SPR methodology has shown an analytical response with API concentrations as low as 0−1 ng/mL, and the LoD for most of HLE-BiTE molecules ranges from 1 ng/mL to 5 ng/mL.
To assess the suitability of SPR technology for evaluating the degradation of HLE-BiTE molecules during cleaning, analysts immobilized protein A and human CD3 on Biacore CM5 sensor chips. Protein A binds to the Fc regions on the HLE-BiTE molecules, whereas the CD3 moiety binds to their anti-CD3 domains. A binding event causes a light-angle change that is captured by a detector and measured in resonance units (RU). My group performed Fc and CD3 binding experiments to assess differences in binding activity of HLE-BiTE protein fragments; only CD3 binding was used in experiments with canonical BiTE molecules because they lack Fc regions that HLE-BiTE molecules have.
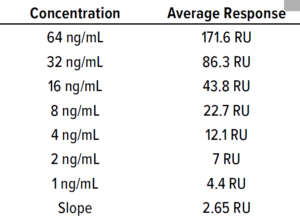
Table 4: Calibration curve data of BiTE
molecule B obtained by the protein A
affinity method; standard error (STEYX) =
0.18, slope = 0.18; limit of detection (LoD) =
0.23 ng/mL, limit of quantitation (LoQ) =
0.7 ng/mL.
Fc Binding Method: A CM5 biosensor chip of the Biacore SPR instrument was immobilized with protein A as a ligand. We built a calibration curve with API sample concentrations of 0−64 ng/mL. The Fc part of the molecule binds to protein A on the chip, then the surface adsorption is flushed for surface regeneration. We tested BiTE-B DS at 64 ng/mL and then serially diluted the solution and tested samples down to 1 ng/mL. The calibration curve in Figure 7 and Table 4 is used to calculate LoD of the SPR method based on standard deviation of the regression line and
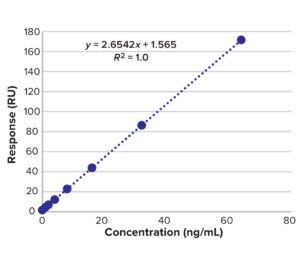
Figure 7: Calibration curve of BiTE molecule B concentration and SPR results.
slope as in Equation 4: LoD = 3.3 SD/m; LoQ = 10 SD/m, where m = slope and SD = standard deviation of the regression line.
Cleaning samples were prepared (Table 5) and tested alongside their corresponding cleaning-matrix samples. Results in Table 6 show that the concentration of cleaning samples — from a manual method, from tangential-flow filtration (TFF) skids, CIP postneutralized (CIP-PN) material, and CIP-PN with steaming — were below the LoD (0.659 ng/mL) of the method. The degradation calculated using the LoD of this method was >99.99% for all cleaning samples.
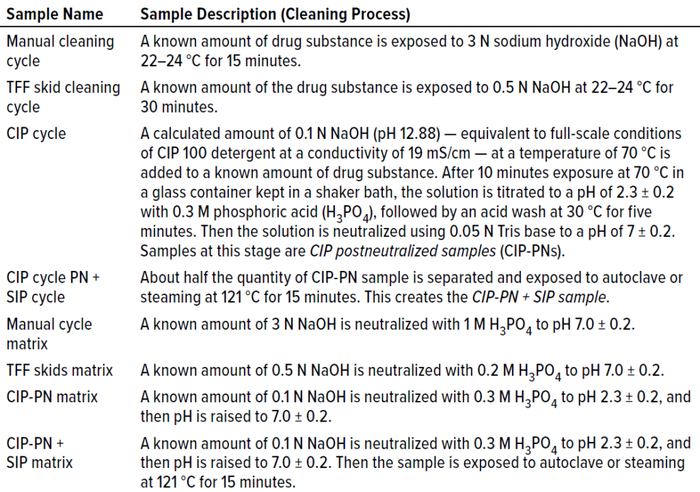
Table 5: Cleaning sample composition and cleaning process for Fc binding experiment;
TFF = tangential-flow filtration, CIP = clean in place, SIP = steam in place.
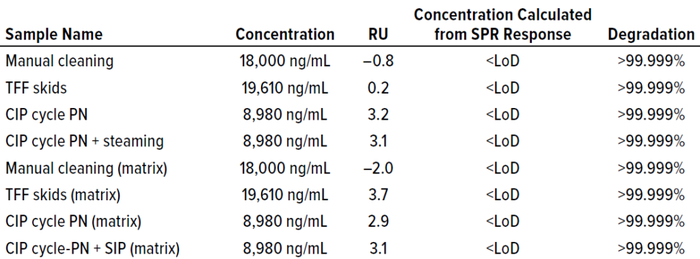
Table 6: Concentration of cleaning samples determined by the Fc binding method.
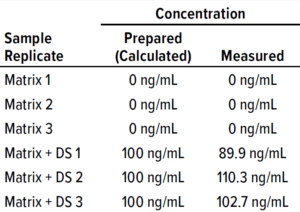
Table 7: BiTE molecule B drug substance
(DS) and cleaning matrix response using
the CD3 binding method.
Human CD3 Binding: For this method, the CD3 proteins are immobilized as ligands on the dextran surface of a CM5 sensor chip. As analytes, injected BiTE and HLE-BiTE molecules bind to the CD3 and cause a light-angle change that is captured by the Biacore instrument’s detector. Then, the biosensor surface is regenerated for the next sample injection.
Figure 7 shows the calibration curve built using untreated BiTE molecule B DS at a concentration of 1 mg/mL,
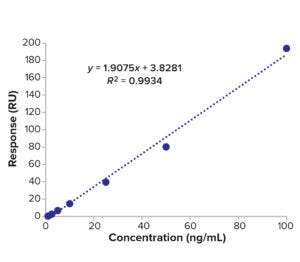
Figure 8: Calibration curve of BiTE molecule B.
which is diluted using 5% intravenous solution stabilizer (IVSS) to 10 μg/mL. Further dilutions were made using 5% IVSS to concentrations of 100 ng/mL and 0.5 ng/mL, as detailed in Table 9 and Figure 8 (10). The diluted samples were tested and their responses recorded in RU (Table 8). We tested the cleaning matrix both with and without DS samples (Tables 7 and 8) to determine whether it would interfere with their SPR responses. Results showed that the cleaning matrix didn’t make a significant difference at 90−110 ng/mL, computed from SPR response compared to the sample concentration (100 ng/mL). Cleaning samples at different sample dilutions (Tables 10 and 11) exhibited some binding activity above the LoD of the method. The positive control sample at 100 ng/mL showed >100% recovery results, and the negative (blank) control sample showed no interference.

Table 8: Sample names and descriptions; IVSS = intravenous solution stabilizer.
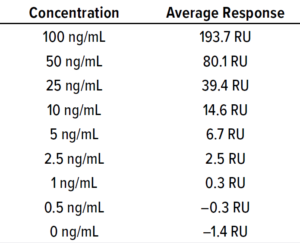
Table 9:BiTE molecule B calibration curve
data from the CD3 method; STEYX = 5.58,
slope = 1.9; LoD = 8.8 ng/mL, LoQ = 29.3
ng/mL.
Sample Filtration To Increase Concentration: The degradation percentage is a function of the difference between cleaning-sample concentration and the sensitivity (LoD) of the analytical method used. Thus, LoD/LoQ is driven by the sensitivity of the method. Increasing the degradation percentage depends on sample concentration. As part of cleaning-process assessment during our bench-scale study, we decreased the API concentration by adding cleaning agents (e.g., caustics) and then acid to neutralize and stop API degradation.
To increase the concentration of the cleaning samples, we used 30-kDa and 50-kDa filters to retain residual protein (API) from the cleaning samples and filter the degraded fragments. Compared with a positive control sample of product without exposure to the cleaning process, the retentate sample would show the API retained on those filters.
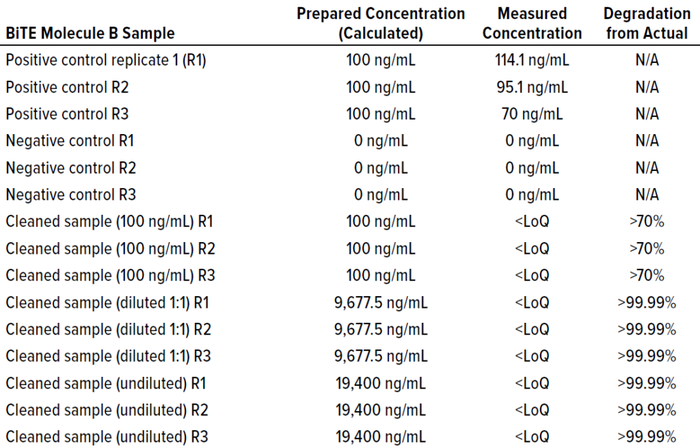
Table 10: BiTE molecule B cleaning samples assessed by the CD3 method.

Table 11: Sample names and descriptions for data in Table 10.
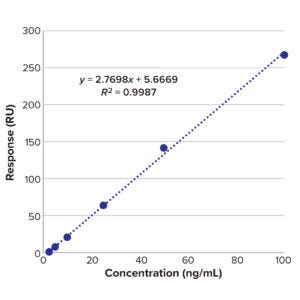
Figure 9: Calibration curve of BiTE molecule B.
Based on the sample volumes retained above the filters, the concentration of undegraded API can be calculated. We use the SPR assay to assess the amount of residual protein (API) in the cleaning samples. Then we can calculate the degradation percentage from the residual amount of protein in the filter-retentate cleaning samples and the calculated value of their protein concentration.
Tables 13 and 16 show the concentration of cleaning samples for BiTE molecules B and C based
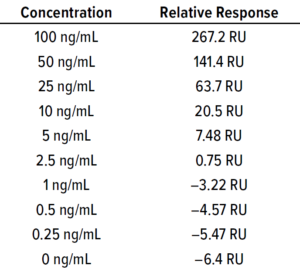
Table 12: Calibration curve data of BiTE
molecule B; STEYX = 3.44, slope = 2.77;
LoD = 4.1 ng/mL, LoQ = 12.41.
on SPR results of the retentate samples and the LoD, the latter being determined using a calibration curve (Figure 9, Table 12). The cleaning-sample results were below the method’s LoD.
Because physical surface adsorption of salts or precipitates that do not bind chemically to the chip can interfere with SPR results, we used a 0.22-µm polyethersulfone (PES) filter to remove salts and precipitates. The positive control sample also was filtered through to ensure that the active DS protein concentration
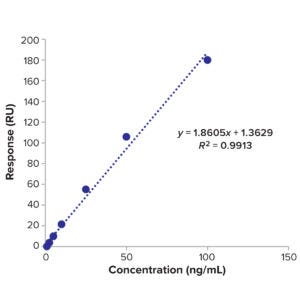
Figure 10: Calibration curve of BiTE molecule C.
would not be affected by this filtration process. Table 14 lists details the different sample preparations of BiTE molecule B, and Table 17 details the different sample preparations of BiTE molecule C. Calibration curve data used in LoD calculations for the two molecules are listed in Table 12 and Figure 9 (molecule B) and Table 15 and Figure 10 (molecule C).
Degradation Percentage Based on Reaction Kinetics (Log Reduction): The sensitivity (LoD) of the SPR method is the lowest concentration that we can measure. Thus, for any concentration below the method’s LoD, we cannot demonstrate or make claims for those concentrations. One option was to assess the rate kinetics of the protein degradation; another option was to use the same log-reduction approach as that used in our sterilization process.
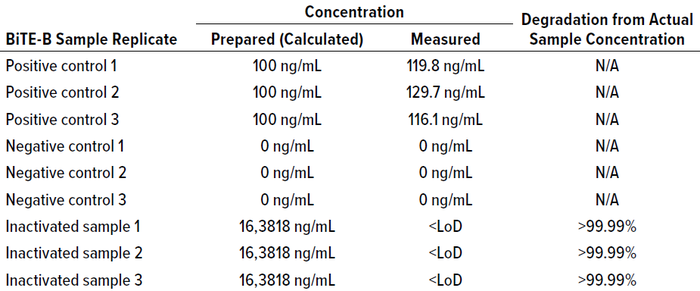
Table 13: BiTE molecule B cleaning samples; inactivated samples are filtered through a
30kDa molecular-weight–cutoff (MWCO) polyethersulfone (PES) filter.
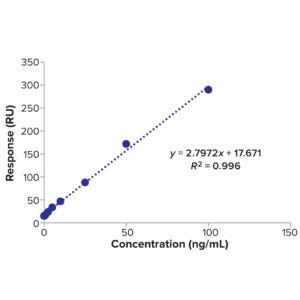
Figure 11: Calibration curve of BiTE molecule B concentration and response.
To assess the reaction kinetics or log reduction (90% degradation), we took cleaning samples at intervals of 30 seconds, one minute, two minutes, and four minutes. The actual full-scale cleaning cycle time was 10 minutes, so 30 seconds represents 5% of the full-scale cleaning cycle time, and one minute represents 10% of it. The degradation percentage of the full cleaning process can be estimated using the percentage of a given period (30 seconds, one minute, two minutes, and four minutes). For reaction (degradation) continuity during the complete cleaning cycle
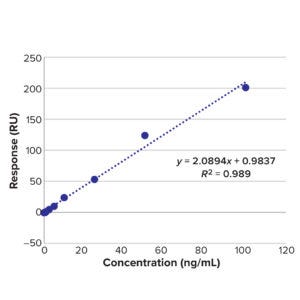
Figure 12: BiTE molecule C calibration curve for concentration and response.
time, we monitor the cleaning conditions of pH and temperature during the cleaning cycle. Calibration curves of known samples used for calculating LoD are shown in Table 18 and Figure 11 (BiTE molecule B) and Table 21 and Figure 12 (BiTE molecule C).
Table 19 shows that BiTE molecule B concentration was below the LoD/LoQ of the SPR method before one minute, indicating >99.999% degradation. Table 22 shows that BiTE molecule C concentration was below the LoD/LoQ before one minute of exposure to the cleaning cycle, thus demonstrating >99.99% degradation within the one-minute cleaning time. Tables 20 and 23 describe the samples tested.
Table 14: Sample names and descriptions for data in Table 13.
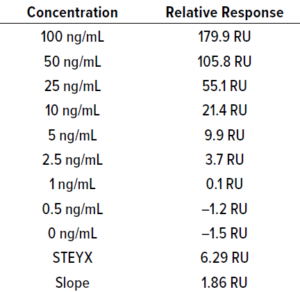
Table 15: Calibration curve of BiTE
molecule C; STEYX = 6.29, slope = 1.86;
LoD = 11.16 ng/mL, LoQ = 33.81 ng/mL.
Increased Sensitivity Improves Safety
The cleaning process described herein demonstrated significant degradation (>99.99%) of two high-potency therapeutic proteins that have extremely low PDE values. Normally, such low values present a critical challenge to the cleaning process for eliminating product carryover risk to ensure patient safety. Application of SPR technology with its high sensitivity to low protein concentrations (1–10 ng/mL) represents a novel approach to demonstrating successful inactivation of highly potent therapeutic proteins for cleaning in multiproduct facilities.
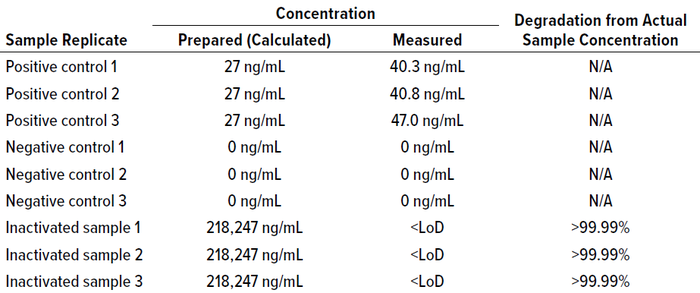
Table 16: Cleaning concentration samples of BiTE molecule C obtained using PES and
30-kDa filters.
Table 17: Sample names and descriptions for BiTE molecule C (Table 16).
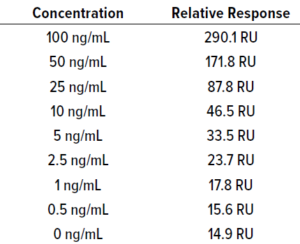
Table 18: BiTE molecule B calibration curve samples; STEYX = 6.48, slope = 2.8; LoD = 6.93 ng/mL, LoQ = 23.11 ng/mL.
Postcleaning samples of BiTE molecules B and C assessed using both protein A binding and CD3 binding SPR methods degraded >99.99% during the cleaning process. The protein A binding method had higher sensitivity compared to the CD3 binding method, although the latter still may be used for both canonical and HLE-BiTE biopharmaceuticals.
By contrast with SPR, SDS-PAGE demonstrates degradation only in the range of 90–95%; for degradation above that range, the degradation-rate approach is used to determine a degradation percentage within 10% of the cleaning cycle. Exposing proteins to 10% cleaning showed >90% degradation (with no residual band observed on the gel). We assessed the degradation percentage using an additional filtration step to increase the concentration of remaining intact active proteins. Based on the increased intact-protein concentration of a positive control sample, the degradation percentage calculated for the cleaning samples was >99.99% degradation.
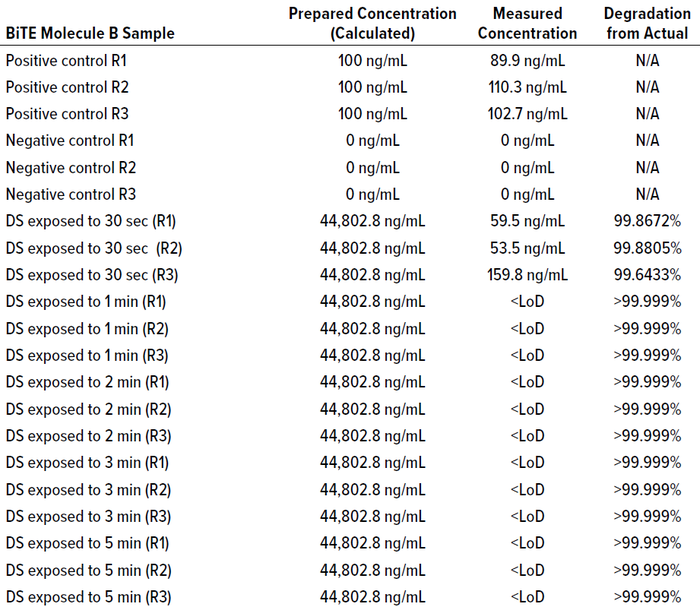
Table 19: BiTE molecule B cleaning sample concentration at different time intervals.

Table 20: Sample names and descriptions of BiTE molecule B for Table 19.
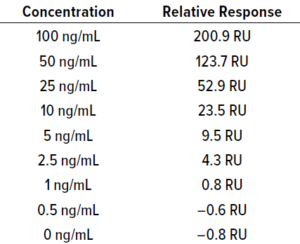
Table 21: BiTE molecule C calibration
curve data; STEYX = 7.92, slope = 2.09;
LoD = 12.52 ng/mL, LoQ = 37.93 ng/mL.
For each cleaning cycle, we collected cleaning samples at time intervals to assess the degradation percentage at different points in the cleaning process. Results showed that within two minutes of a 10-minute CIP 100 detergent step, the residual therapeutic protein concentration was below the SPR method’s LoD (thus >99.99%), which was sufficient to demonstrate that the PDE was achieved. During a complete cleaning cycle of 10 minutes, the degradation will be significantly greater than >99.9999% as long as the cleaning conditions of pH and temperature are maintained.
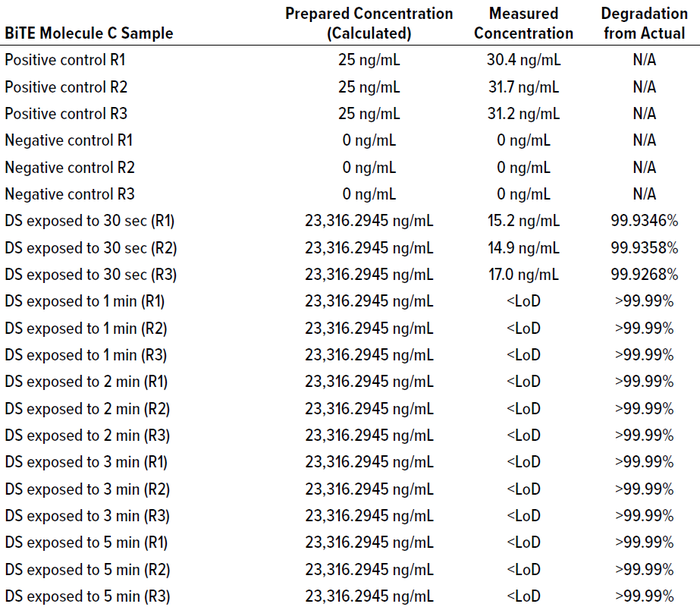
Table 22: BiTE molecule C cleaning-sample concentration and degradation at different
time intervals.
Table 23: Sample names and descriptions for BiTE molecule C samples in Table 22.
References
1 Chapter 3: Premise and Equipment. Good Manufacturing Practice (GMP) Guidelines: Part I — Basic Requirements for Medicinal Products. EudraLex Volume 4. European Commission: Brussels, Belgium, 2008; https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-4/pdfs-en/cap3_en.pdf.
2 Fourman GL, Mullen MV. Determining Cleaning Validation Acceptance Limits for Pharmaceutical Manufacturing Operations. Pharm. Technol. 17(4) 1993: 54–60.
3 EMA/CHMP/ CVMP/ SWP/169430/2012. Manufacture of Different Medicinal Products in Shared Facilities. European Medicines Agency: London, UK, 20 November 2014; https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-setting-health-based-exposure-limits-use-risk-identification-manufacture-different_en.pdf.
4 PI 046-1 Annex. Guideline on Setting Health Based Exposure Limits for Use in Risk Identification in the Manufacture of Different Medicinal Products in Shared Facilities. Pharmaceutical Inspection Cooperation Scheme: Geneva, Switzerland, 1 July 2018: https://www.picscheme.org/layout/document.php?id=1412.
5 Sharnez R, et al. Cleaning Validation Acceptance Limits for Biological Process Residues: Part 1 — Acceptable Exposure of Degraded Proteins Based on Reference Immunogens. BioProcess Int. 21(5) 2023: 26–31, 43; https://bioprocessintl.com/category/may-2023/cleaning-validation-acceptance-limits-for-biological-process-residues-part-1.
6 Thode J. How To Determine the LOD Using the Calibration Curve? Lösungsfabrik Blog 20 June 2019; https://mpl.loesungsfabrik.de/en/english-blog/method-validation/calibration-line-procedure.
7 Chromatographic Measurements, Part 5: Determining LOD and LOQ Based on the Calibration Curve. Separation Science https://blog.sepscience.com/liquidchromatography/hplc-solutions-126-chromatographic-measurements-part-5-determining-lod-and-loq-based-on-the-calibration-curve.
Corresponding author Ram Kouda is a principal scientist, Syeda Tabassum is a senior associate scientist, and David Dolan is a senior principal scientist at Amgen, 1 Amgen Center Drive Thousand Oaks CA 91320; [email protected].
You May Also Like