Content Spotlight
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
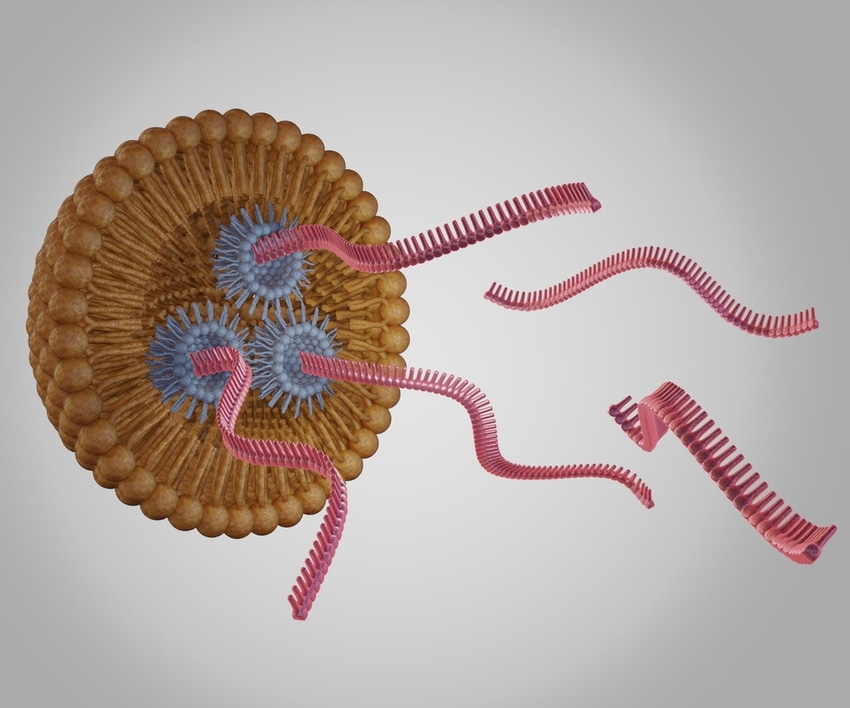
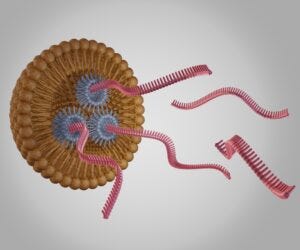
GenScript Biotech adds 400,000 square-feet of single RNA and non-viral DNA manufacturing capacity at its site in Zhenjiang, China to support biopharma cell and gene therapy (CGT) projects.
The recently operational five-story 400,000 square-foot plant in Zhenjiang offers multiple segregated manufacturing and purification cleanrooms, Kay Huang, head of marketing at GenScript Biotech USA, said. “This new expansion will allow us to deliver GMP materials faster and help expedite our clients’ project timelines.” No financial details were divulged.
“The demand come from clients using CRISPR technology or other gene-editing tools for developing gene and cell therapeutics,” we were told.
DepositPhotos/[email protected]
“For gene insertion, correction or replacement, CRISPR sgRNA [single RNA] and payloads for gene delivery are needed. With the rapid development we are seeing in this field, more projects are moving into the clinical phase, yet the availability of cGMP reagents are limited. The goal of these new expanded lines is to help gene- and cell-therapy clients move their research into clinical studies faster.”
The facility complements the five manufacturing lines at its facility in Nanjing, established by GenScript in 2021.
“Our cGMP capabilities are now focused on supporting CRISPR-based CGT [cell and gene therapy] applications — for example CAR/TCR-T researchers, CRISPR-based gene therapies, and more. As a cGMP sgRNA provider, we have delivered 56 batches as of April 2023, supported dozens of project applications, five of which have received IND approvals,” Huang told this publication.
The Zhenjiang expansion increases GenScript’s position in the fadvanced therapies contract development and manufacturing organization (CDMO) space.
“With the new expansion, we are now able to provide a faster than ever cGMP sgRNA service to our clients. As for non-viral payloads (ssDNA, linear closed-end dsDNA, and circular miniature dsDNA), we have been leading the field and its development since 2017, when we started collaborating with world leaders in the cell therapy field (for example, the Marson Lab at UCSF), in testing ssDNA in CAR-T development.
“To our knowledge, we are the only vendor that can provide cGMP sgRNA and non-viral payloads in multiple formats at the same time. This gives our clients the flexibility they need to explore and optimize their ideal approach, and to source everything from research use to cGMP from one vendor.”
You May Also Like