Leveraging Plant-Based Protein Expression: Implications for Biomanufacturing and Biodefense

A scientist performs agroinfiltration of Nicotiana benthamiana leaves. (CHANDRES, HTTPS:// COMMONS.WIKIMEDIA.ORG/WIKI/FILE:AGROINFILTRATION.JPG)
Although a 1972 United Nations (UN) resolution prohibits member states from using biological weapons on military personnel and civilians (1, 2), a lack of robust verification protocols has enabled several countries to develop such agents (3). Several groups that the UN considers to be global terrorist organizations also are establishing bioweapon capabilities (4). Meanwhile, potentially pandemic infectious diseases continue to pose public-health threats. Consequently, research remains active in vaccine and therapeutic development for bioterrorism mitigation and other biodefense applications.
Recombinant antibodies (rAbs) represent a relatively new and highly promising set of tools in that context. In a 2011 review, Froude et al. explain that such products could bolster biosecurity in several ways (3). Because rAbs have different mechanisms of action (MoAs) from those of antibiotics, rAb products circumvent concerns about bioagents that are engineered for antimicrobial resistance. In some cases, antibodies could provide prophylactic effects despite their relatively short half-lives. And if nothing else, rAbs could work synergistically with existing small-molecule treatment regimens.
When Froude et al. reviewed the drug-development landscape for biodefense-related rAbs in 2011, several clinical and commercial advances already had been made for treating Bacillus anthracis (anthrax) exposure. The writers attribute those successes, in part, to significant US-government investment in biodefense following the mailing of anthrax spores to members of the US Congress and news media in 2001. However, “the situation regarding anthrax has no equivalent so far, perhaps because oligoclonal rAbs may be necessary to effectively target a single agent, such as Yersinia pestis or Burkholderia pseudomallei, thus increasing the complexity of antibody development.”
As today’s biopharmaceutical scientists know, drug developers and manufacturing partners also need to negotiate hurdles relating to production speed and cost, both of which are critical factors when responding to biosecurity threats. Antibody-manufacturing processes based on Chinese hamster ovary (CHO) and other mammalian cell lines can take considerable time and resources to develop, optimize, and perform. Yet such expression systems dominated development pipelines in 2011: Froude
et al. highlight that nearly all of the candidates that they identified were expressed by murine systems (3). That trend continues across today’s biopharmaceutical industry.
However, as I learned from Don Stewart, alternative protein-expression systems are coming into their own, especially because of their potential advantages for production speed, cost, and scalability. Stewart is a biotechnology entrepreneur with a doctoral degree in biochemistry from the University of London, UK, and over 20 years of managerial experience in the industry. He cofounded and now serves as chief executive officer of PlantForm Corporation in Toronto, ON, Canada, as well as its wholly owned subsidiary AntoXa Corporation. Both companies leverage plants to express protein therapeutics, and AntoXa specializes in medical countermeasures to biological and chemical agents. Among its clinical programs is PhD9, a monoclonal antibody (MAb) for treatment of ricin poisoning. The program was developed with support from the Medical Countermeasure Consortium (MCMC) under the Chemical, Biological and Radiological Memorandum of Understanding (CBR MOU) among Australia, Canada, the United Kingdom, and the United States. The US Centers for Disease Control and Prevention (CDC) classify ricin as a category B biological agent, placing it among high-priority toxins and pathogens that are “moderately easy to disseminate, result in moderate morbidity rates and low mortality rates, and require specific enhancements of CDC’s diagnostic capacity and enhanced disease surveillance” (5). Of particular concern is that ricin can be aerosolized.
In October 2023, Stewart and I spoke about ricin as a biosecurity threat, the utility of MAb-based bioterrorism countermeasures, and the role that plants might play in developing such therapeutics. He explained how plant-based systems enable flexible, high-yield, and low-cost manufacturing of proteins with the requisite glycosylation profiles. He also emphasized that drug developers have overcome many of the technical and regulatory concerns that hampered initial research into plant-system protein expression. Now, the goal is to increase developer access to large-scale biomanufacturing capacity.
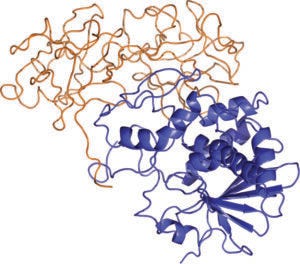
Ribbon diagram of ricin, with A and B chains shown in blue and orange, respectively (AZA TOTH, HTTPS://COMMONS. WIKIMEDIA.ORG/WIKI/FILE:RICIN_STRUCTURE.PNG)
Treating Ricin Exposure
What chemical features are distinctive to ricin, and why is it especially toxic in humans? Ricin is expressed naturally in castor beans, from which it is relatively easy to purify. When circulating in a patient’s bloodstream, intact ricin proteins are nontoxic; their toxicity manifests when they are internalized by cells. Ricin comprises two glycoprotein chains (A and B) joined by a disulfide bond. The B chain can bind to cell-surface antigens, enabling the molecule’s uptake. Cellular processes disrupt the disulfide bond and release the A chain, which functions as a ribosome-inactivating protein, or an RIP — a fitting acronym considering how deadly such molecules are. Ribosomes perform protein synthesis. A-chain release disrupts that process, leading to the poisonous effects that a patient experiences. (Editor’s note: Ricin inhalation can cause respiratory distress, fever, cough, and nausea, leading into heavy sweating, pulmonary edema, low blood pressure, and respiratory failure (6). Ingesting ricin can cause vomiting and diarrhea followed by severe dehydration, bloody urine, seizures, and failure of critical organs.) Intact ricin molecules have extremely high affinity for cell-surface targets. That feature makes the toxin especially concerning because it is very quick and effective at creating its toxic effects.
Why might MAbs present a good strategy for treating ricin exposure? In principle, what must a MAb do to elicit a therapeutic response? I know of several organizations that have tried to produce neutralizing monoclonal antibodies (NAbs) for treatment of ricin exposure. Scientists at the Suffield branch of Defence Research and Development Canada (DRDC) started that kind of work years ago (7). The agency produced a large number of murine-expressed antibodies, some of which neutralized ricin. Then, the organization selected a couple of candidates for advancement, one of which functioned particularly well as a NAb.
We believe that our candidate MAb forms complexes with intact ricin, blocking it from binding to cell-surface markers. Specifically, the antibody may span the junction of the A and B chains, preventing the conformational changes required for binding. So complexed ricin molecules do not enter into patient cells, preventing formation of standalone A chains and their associated toxicity. We are still studying our MAb’s binding interactions.
A Primer in Plant-Based Biomanufacturing
I know that your company uses plant-based systems to express the candidate MAb. What host organism do you use, and more generally, what systems are available for such applications? We use Nicotiana benthamiana plants, which are the most frequently used host in the industry for plant-based production. Other systems under evaluation include varieties of maize, rice, lettuce, and potato (8). But the vast majority of operators in our field use N. benthamiana, which is not a food plant. And rather than growing stable, transgenic plants that produce a given product, most developers perform transient expression, introducing a gene of interest (GoI) to plant hosts using an Agrobacterium vector (9).
Why has N. benthamiana become the industry’s preferred platform for plant-based protein production? Does the organism have particularly useful features for such processes, or do researchers simply have a wealth of information about it? Several factors are involved. N. benthamiana leaves are much less waxy than those from other tobacco varieties. Compared with other strains that have been studied, N. benthamiana generates low levels of proteases, nicotine, and other impurities. Scientists also know a good deal about the molecular biology and genetics of another Nicotiana strain (tabacum) because of research performed by tobacco companies. Equally important is that N. benthamiana grows well. It is naturally protected against insects and other pests, and it grows easily, safely, and inexpensively in greenhouses and vertical farming systems.
AntoXa has developed a five-week production process (Figure 1). We grow N. benthamiana plants for four weeks before introducing a GoI through an Agrobacterium species; then, the plants continue growing for another week before we harvest material. Over five weeks, the plants grow to about the size of a lettuce, so we get a large amount of plant biomass from a small footprint. If you are concerned about cost of production, then benthamiana is the route to take because it can generate a large amount of biomass per square foot of facility space, and you can stack production systems vertically for greater output.
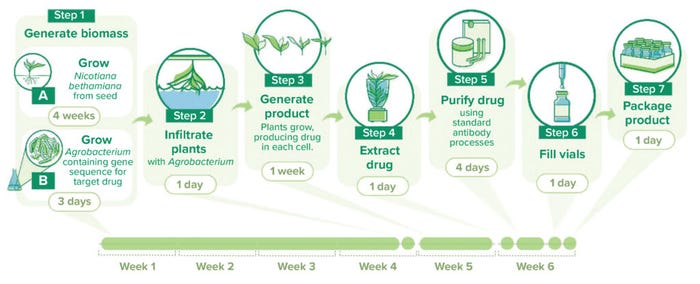
Figure 1: A sample workflow for plant-based protein manufacturing in Nicotiana benthamiana using PlantForm Corporation’s vivoXPRESS platform.
An added advantage is that the plant is amenable to genetic modifications from agrobacteria vectors. That feature lends itself well to producing stable transgenic plants with improved characteristics.
What advantages might plant-based platforms — N. benthamiana or otherwise — have over conventional mammalian- and microbial-cell platforms? Using plants is less expensive; that is the main advantage. Production processes account for a considerable part of costs for CHO-based therapies (10). If you are targeting price-sensitive markets — such as those for biosimilars and, in our case, biodefense — then using a low-cost manufacturing system makes sense.
Facilities for plant-based protein manufacturing also are much easier and less expensive to establish than are facilities for mammalian-cell production. That is primarily because plants are not grown in large bioreactors using highly specialized media. CHO-based production also involves ancillary expenses for steam, water, and other materials for equipment sterilization at the production stage, which plant-based manufacturing does not. As with all biologics, plant-based drug substances do require sterile processing during purification, formulation, and filling.
How would you characterize a typical workflow for manufacturing your ricin-binding MAb? The first step is to generate biomass. We seed the plants in trays, a process that can be automated at large scale. Then, the plants are grown for four weeks. In a laboratory/development context, we can grow plants in greenhouses or use single-layer contained growth. But in a manufacturing environment, we use vertical farming. Environmental controls are simple: Because the process is hydroponic, we control temperature, humidity, and carbon-dioxide levels. Plant growth in such formats is simple and well characterized.
Toward the end of that four-week cycle, we generate the agrobacteria bearing the GoI and control mechanisms that affect glycosylation and expression rate. That process takes about three days.
Next, we perform infiltration, diluting the agrobacteria to a low concentration in a buffer and inverting trayed plants into that solution. The process occurs in a vacuum chamber so that we can draw air out of the plants’ leaves. When we release the vacuum, the leaves backfill with agrobacteria solution. Using natural mechanisms, the microbes infect the plants.
We have taken over that mechanism. Upon infecting a plant, such bacteria usually create galls. We have changed the microbes’ molecular biology so that infiltrated plants produce a protein of interest with the correct glycosylation profile. Modifying the agrobacteria also improves production rates.
Next, we generate the MAb product. After infiltration, we grow the plants in the same vertical farming environment for about one week, then cut them down at soil level. The plants are mashed up with a mill and sonicated. Residual plant material is separated from the product-containing liquid by filtration, and the filtrate undergoes a standard biopharmaceutical purification process.
What process to use depends on the application. For injectable drugs for human use, our purification process involves protein A capture followed by two orthogonal steps for removing residual contaminants and endotoxins. We developed that process on the basis of good science when we were producing a Herceptin (trastuzumab) biosimilar. The final step is crossflow filtration through a 0.2-µm membrane. On the other hand, drugs for animal-feed purposes might require little to no processing. You can perform nutraceutical-grade processing, which requires less purity than what is needed for human parenteral products. Alternatively, you can use the leaves themselves. For one of our programs, we grind dry product-bearing leaves, creating a powder that can be top-dressed onto pig feed.
Flexible, Well-Controlled Gene Expression
How amenable are Agrobacterium and Nicotiana cells to gene editing? And what special considerations arise in designing gene sequences for plant-based protein systems? If the goal is to create transgenic plants, then standard gene-editing methods can be applied. For instance, we use clustered regularly interspaced palindromic repeat (CRISPR) technology.
For transgenic approaches, expression rate is particularly important because it affects manufacturing costs. PlantForm initially licensed a PD9 promoter system to help control expression rate (11). We have since created our own system based on Argonaute proteins, which were developed initially for yeast-based protein expression (12). Establishing our own system has reduced production costs.
The process is different when using agrobacteria because you do not edit the genes of the plants; you introduce an infection that can be used to deliver a GoI so that plant hosts will create the product of that gene. We use electroporation to introduce gene sequences into agrobacteria and codon-bias the genes for plant production. Usually, we remove the introns, but those can be reinserted when doing so has advantages for production. What gene-editing strategy to use is a decision made case by case, striking a balance between codon bias and intron use.
Depending on what PTMs are needed, agrobacteria can be designed to direct products into different plant-cell compartments — e.g., chloroplasts, vacuoles, cytoplasm, and apoplasts. The microbe can deliver up to 10 genes into a plant cell at a time. Through electroporation, we give the agrobacteria genes not only for expression of a MAb product, but also for PTMs of glycans, proteases, and amino acids. We also have a product for which we modify agrobacteria to deliver mechanisms for proline hydroxylation so that we can optimize expression rates. That is important to minimize costs.
Plant-based systems provide a large amount of control and flexibility simultaneously, enabling you to obtain a product in a desired format. That is a key advantage of plants over other production systems. You do not always have both control and flexibility built into your production method when using CHO or other mammalian cells.
The Path Ahead for Plant Systems
My impression is that plant expression systems have tended to generate low product yields and sometimes high proportions of proteins with diminished biological activity (13). How have drug developers addressed such concerns? Most such concerns have been resolved. When scientists started using plants for protein expression, they focused on transgenic plants thinking that doing so would be easy: You engineer your seeds, then plant and grow them. But transgenics tend to have low production rates.
About 30 years ago, researchers started considering transient production using N. tabacum. That approach did not work particularly well. Those plants have difficult-to-manage structures because their leaves are quite waxy. They also generate relatively little biomass in small footprints. They grow tall and are fairly slow to grow. Once research into other Nicotiana strains began, scientists found that N. benthamiana allowed both easy introduction of agrobacteria and high growth and protein-expression rates in a short timeframe. I believe that the case for plants has been made now, as demonstrated by current high-yield, low-cost bioproduction processes.
Another consideration is improved modification of plant-cell glycosylation structures. Plant and mammalian cells are eukaryotes, but they have different glycosylation patterns. PlantForm/AntoXa and other organizations in the plant-production space have modified host-cell glycosylation to eliminate plant-specific glycosylation and bring in aspects of glycosylation that are missing from mammalian cells. PlantForm initially did that work with transient-production platforms, but now we also can generate transgenic hosts with favorable characteristics. We can achieve consistent production of products with mammalian glycosylation patterns using either transgenic plants or a combination of transgenic hosts and transient inserts of enzymes for glycosylation-pathway modification. Both strategies are advantageous because they enable simple control of glycosylation. Glycosylation control is much more difficult with mammalian cells and microbes (e.g., Pichia pastoris).
What does the biopharmaceutical industry need to make plant-based protein expression more common? The key consideration now is investment in manufacturing infrastructure. Specifically, there is need for large-scale biomanufacturing capacity. A primary reason for not adopting plant-cell technology, I believe, is that drug developers cannot easily find an array of contract manufacturing organizations (CMOs) from which to choose. A limited number of CMOs operate in our space.
Companies such as PlantForm/AntoXa are encouraging governments to invest in such infrastructure, either directly or through matching programs. I believe that government funding and capital from large pharmaceutical companies for biomanufacturing capacity will be critical to moving plant-cell–production programs forward. Such advances are happening. Japanese company Denka has acquired Icon Genetics in Germany to build large-scale manufacturing capacity for plant-based biologics. PlantForm is involved in a project with Brazil’s Ministry of Health, which plans to establish a large-scale facility in that country. The Canadian government is exploring similar projects with drug developers, including PlantForm.
Do any technical or regulatory challenges still need to be resolved for plant-expressed biologics to take root in the industry? I don’t believe so. Companies such as ours are leveraging considerable molecular-biology research from the tobacco industry. We are using proven plant-growth technology from the vertical-farming food industry. Extraction of products from plants is a well-established process from the food-production industry. And we are purifying products according to standard biopharmaceutical processes.
Regulatory authorities also have given marketing approval to some drugs manufactured in plant systems (14, 15). For a long time, detractors would say, “We need to see a regulator-approved injectable product before we can believe in the viability of plant-based systems.” I once spoke to a US Food and Drug Administration (FDA) representative about that point, asking about the agency’s position on plant-based expression systems. The message that I received was that the agency does not care per se about what system is used to produce a drug. Rather, a biomanufacturer must demonstrate that a product is safe and efficacious — the onus that is placed on any manufacturer.
Plants have been used to make drugs for many years. Originally, aspirin was derived from willow, and raw materials for several chemotherapy agents come from plants. Regulatory agencies are quite familiar with plant biology. The barrier had been that companies in our space were using plants in a novel way, making large-molecule, recombinant products. That was the step that had to be taken, and now that regulators have granted approval for some plant-based recombinant proteins, companies such as PlantForm are comfortable with regulatory expectations.
What advice do you have for other companies that are interested in pursuing plant-based expression systems? Do you find the approach especially advantageous for biosecurity applications, or is it viable for more general pharmaceutical development? I believe that plant-cell systems can serve both applications. For biosecurity contexts, plant-expression technology produces biologics quickly and at low costs. Those are exactly the criteria that you need to meet when developing bioterrorism countermeasures. An added benefit is that new facilities for plant-based protein production can be established in short order by leveraging greenhouses and/or vertical-farming units, the latter of which are commercially available as “off-the-shelf” modules.
Regarding pharmaceutical development more generally, I believe that plant-based expression systems can be especially advantageous for contexts requiring low-cost biopharmaceuticals. I know that some companies are using plant systems to express insulin (16). That is an interesting case because insulin is basically a commodity with highly competitive pricing across manufacturers. But I believe that plant-based technology will be particularly advantageous for producing biosimilars, for which there is significant pressure on price control. PlantForm already is exploring such applications. The high prices of innovator drugs raise significant barriers to adoption by healthcare systems. Consider that immunotherapy with Keytruda (pembrolizumab) has shown much success, but treatment (at least initially) cost US$150,000 (17). Lowering manufacturing costs makes sense in such cases.
References
1 Henderson DA. The Looming Threat of Bioterrorism. Science 283(5406) 1999: 1279–1282; https://doi.org/10.1126/science.283.5406.1279.
2 Treble A. Chemical and Biological Weapons: Possession and Programs Past and Present. Center for Nonproliferation Studies: Monterey, CA, 2002.
3 Froude JW, et al. Antibodies for Biodefense. mAbs 3(6) 2011: 517–527; https://doi.org/10.4161/mabs.3.6.17621.
4 Sugishima M. Aum Shinrikyo and the Japanese Law on Bioterrorism. Prehosp. Disaster Med. 18(3) 2003: 179–183; https://doi.org/10.1017/s1049023x00001023.
5 Bioterrorism Agents/Diseases. US Centers for Disease Control and Prevention: Atlanta, GA, 4 April 2018; https://emergency.cdc.gov/agent/agentlist-category.asp.
6 Facts About Ricin. US Centers for Disease Control and Prevention: Atlanta, GA, 4 April 2018; https://emergency.cdc.gov/agent/ricin/facts.asp.
7 Hu WG, et al. Humanization and Characterization of an Anti-Ricin Neutralization Monoclonal Antibody. PLoS One 7(9) 2012: e45595; https://doi.org/10.1371/journal.pone.0045595.
8 Cossar D, McLean MD, Stewart D. Plant-Based Protein Expression: Emerging Systems Bring Viable Approaches to Biopharmaceutical Manufacturing. BioProcess Int. 20(5) 2022: 32–37; https://bioprocessintl.com/upstream-processing/expression-platforms/plant-based-protein-expression-emerging-systems-bring-viable-approaches-to-biopharmaceutical-manufacturing.
9 Uthailak N, et al. Transient Production of Human β-Glucocerebrosiade with Mannosidic-Type N-Glycan Structure in Glycoengineered Nicotiana benthamiana Plants. Front. Plant Sci. 12, 2021: 683762; https://doi.org/10.3389/fpls.2021.683762.
10 Yang O, Qadan M, Ierapetritou M. Economic Analysis of Batch and Continuous Biopharmaceutical Antibody Production: A Review. J. Pharm. Innovation 15, 2020:
182–200; https://doi.org/10.1007/s12247-018-09370-4.
11 Jiang P, et al. Characterization of a Strong and Constitutive Promoter from the Arabidopsis serine Carboxypeptidase-Like Gene AtSCPL30 As a Potential Tool for Crop Transgenic Breeding. BMC Biotechnol. 18, 2018: 59; https://doi.org/10.1186/s12896-018-0470-x.
12 Wu J, et al. Argonaute Proteins: Structural Features, Functions and Emerging Roles. J. Adv. Res. 24, 2020: 317–324; https://doi.org/10.1016/j.jare.2020.04.017.
13 Liu H, Timko MP. Improving Protein Quantity and Quality — The Next Level of Plant Molecular Farming. Int. J. Mol Sci. 23(3) 2022: 1326; https://doi.org/10.3390/ijms23031326.
14 Fox JL. First Plant-Made Biologic Approved. Nature Biotechnol. 30(6) 2012: 472; https://doi.org/10.1038/nbt0612-472.
15 Medicago Covifenz COVID-19 Vaccine. Health Canada: Ottawa, Ontario, 31 March 2022; https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/drugs-vaccines-treatments/vaccines/medicago.html.
16 Alyas J, et al. Human Insulin: History, Recent Advances, and Expression Systems for Mass Production. Biomed. Res. Ther. (Vietnam) 8(9) 2021: 4540–4561; http://dx.doi.org/10.15419/bmrat.v8i9.692.
17 Weintraub A. Merck’s Melanoma “Game-Changer” Keytruda Likely To Bolster Drug Pricing Debate. Fierce Pharma 5 September 2014; https://www.fiercepharma.com/pharma/updated-merck-s-melanoma-game-changer-keytruda-likely-to-bolster-drug-pricing-debate.
Brian Gazaille, PhD, is managing editor of BioProcess International; [email protected]; 1-212-600-3594. Don Stewart, PhD, is cofounder and chief executive officer of PlantForm Corporation and AntoXa Corporation.
You May Also Like





