Anion-Exchange Chromatographic Clarification: Bringing Simplification, Robustness, and Savings to MAb Purification
 Monoclonal antibodies (MAbs) are the most prominent and successful therapeutic proteins in the pharmaceutical industry. More than 35 MAbs have been approved to treat a range of conditions, and hundreds more are in development (1, 2). Once, the upstream cell culture process was considered the bottleneck to producing high antibody doses required for treatment, but recent advances in cell culture technology have boosted antibody titers to the range of 5–10 g/L (3). That increase in productivity has shifted focus onto downstream antibody purification bioprocess development (1, 4). In addition to advancements in cell- line development, the now-typical higher antibody titers have been achieved by optimizing cell culture media and growth conditions, which increases cell culture density and lengthens culture duration. That translates into higher levels of process-related impurities such as host-cell proteins (HCPs) and DNA, lipids, colloids, and cell debris (1). The main goal of a downstream process (DSP) is to reduce process- and product-related impurities to acceptable levels (2, 3). Higher impurity levels present challenges across a DSP from primary recovery through capture and polishing chromatography steps.
Monoclonal antibodies (MAbs) are the most prominent and successful therapeutic proteins in the pharmaceutical industry. More than 35 MAbs have been approved to treat a range of conditions, and hundreds more are in development (1, 2). Once, the upstream cell culture process was considered the bottleneck to producing high antibody doses required for treatment, but recent advances in cell culture technology have boosted antibody titers to the range of 5–10 g/L (3). That increase in productivity has shifted focus onto downstream antibody purification bioprocess development (1, 4). In addition to advancements in cell- line development, the now-typical higher antibody titers have been achieved by optimizing cell culture media and growth conditions, which increases cell culture density and lengthens culture duration. That translates into higher levels of process-related impurities such as host-cell proteins (HCPs) and DNA, lipids, colloids, and cell debris (1). The main goal of a downstream process (DSP) is to reduce process- and product-related impurities to acceptable levels (2, 3). Higher impurity levels present challenges across a DSP from primary recovery through capture and polishing chromatography steps.
Although the process for manufacturing each commercial antibody is unique, MAb processes generally share a number of key features. Antibodies are expressed by mammalian cell cultures grown in suspension in batch-fed bioreactors (2). The first step in downstream processing is clarification of cell-culture fluid containing a target antibody. Primary recovery traditionally is accomplished through the use of disk- stack continuous centrifugation followed by depth filtration and sterile filtration (1). Purification requires multiple chromatography steps — generally one capture step (e.g., protein A or cation- exchange chromatography) and two polishing steps — with additional filtration and viral clearance unit operations (2, 5).
Important ImpurItIes Protein A affinity chromatography is the preferred capture step because of protein A’s highly specific interaction with antibodies. Typically, this step yields >95% product purity (1, 6). Remaining impurities have significant economic ramifications and often require two or three additional polishing chromatography steps for reduction to acceptable levels (6). The prevalence of protein A capture in MAb purification, the significant cost of protein A resins, and downstream challenges associated with impurities present in the resulting fraction have driven extensive research to identify those contaminants and the nature of the interactions that prevent protein A resins from providing higher product purities. HCPs and chromatin heteroaggregates are two primary impurity candidates, and their associations with MAbs and protein A resins are the subjects of several studies (6, 10, 12–14).
Host-Cell Protein: HCPs constitute a major component of process-related impurities with the potential to elicit adverse immune reactions in patients (7). Heterogeneity in the physical and biological properties of HCPs makes them particularly difficult to remove, requiring multiple unit operations to do so (8). The profile of HCPs that need to be removed during downstream processing is influenced by expression conditions and primary recovery operations. Studies of centrifugation and depth filtration indicate that the approach used during primary recovery influences the HCP profile, not only in the feedstock before protein A capture, but also in eluate following the capture step (8, 9).
Two major mechanisms have been reported that explain the presence of HCPs in protein A pools. The first one is “hitch- hiking,” which refers to strong associations among certain HCPs, DNA, and MAbs. HCP species bound to a product are carried out during elution of a protein A column (6, 10). The second mechanism is coelution, in which HCPs bind to the protein A ligand or resin matrix and are released during product elution (11).
Chromatin is gaining attention because it can significantly affect the performance of chromatography columns. Chromatin is a complex macromolecular assembly — comprising DNA, RNA, and proteins (primarily histones) — found in eukaryotic cells (12). Recent research by Gan et al. suggests that chromatin heteroaggregates released from dead cells form strong associations with both IgG and IgM (13). Such associations can lead to hitch-hiking of the impurities (DNA, HCP) during protein A elution (13, 14).
Gagnon et al. demonstrated that chromatin binds to the surface of protein A resins, reducing their dynamic binding capacity (DBC) by about 20% (12). Moreover, chromatin heteroaggregates bound to protein A resin may become binding sites for other contaminants. Dissociation of the interactions between chromatin complexes and contaminants under elution conditions may lead to higher levels of impurities in the protein A fraction. In the same study, 99% of contaminants present in a protein A fraction were the results of leaching from resin-bound chromatin aggregates under elution conditions (12).
Those research findings suggest that a purification strategy capable of removing chromatin heteroaggregates, HCPs, and associated impurities from the cell cultures before protein A chromatography would remarkably increase protein A performance efficiency. That would ultimately lead to higher product purity. Previous attempts to implement such a strategy have been hindered by a lack of chromatography technologies that can be applied at the clarification stage. Such a technology would need to be capable of handling the large process volumes, high DNA and HCP loads, and high stream turbidity that are typical in cell culture supernatant.
Here, we investigate a strategy that uses a novel chromatographic clarification enabled by 3M’s Emphaze AEX Hybrid Purifier capsules. They address the challenges mentioned above by significantly reducing both particulates and soluble contaminants (such as DNA and HCPs) early in primary recovery. The capsules are designed to be used at the end of the primary recovery stage of mammalian cell cultures, following either centrifugation or coarse depth filtration.
A New Approach to Clarification
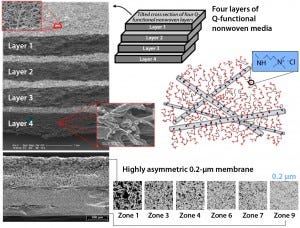
An Emphaze AEX Hybrid Purifier chromatographic clarification capsule is a unique single-use product that is distinctly different in construction and mode of operation from depth filters, chromatography columns, and membrane adsorbers. It combines three technologies — anion-exchange (AEX) functional polymers, fine-fiber nonwoven materials, and multizone membranes — to deliver an all-synthetic clarifying product containing both a novel AEX hydrogel medium and a fine-particle and bioburden-reduction membrane. A quaternary ammonium (Q) functional hydrogel — roughly two-thirds by mass an AEX functional polymer covalently grafted to a fine-fiber polypropylene nonwoven scaffold — provides both mechanical sieving and electrostatic capture of negatively charged cell debris. The AEX capacity of that medium significantly exceeds that of conventional depth filters, providing substantially higher reduction of soluble anionic impurities including DNA and anionic HCPs.
Inclusion of a high-capacity, nine-zone membrane with a 0.2-µm–rated qualifying zone makes these flow- through chromatography capsules the only clarifying devices with a defined pore size. That provides an ensured, high level of turbidity reduction in clarification as well as >6 log reduction value (LRV) bioburden reduction. As part of 3M’s single-use capsule family, these capsules come in sizes that are appropriate for use at laboratory, pilot, and manufacturing scales.
Because of its unique properties, using flow-through chromatography as part of a clarification strategy produces a highly purified clarified cell-culture fluid (CCCF). Such purity offers significant benefits that extend throughout downstream processing. Providing a significantly cleaner CCCF stream for downstream purification, AEX chromatographic clarification capsules provides critical process benefits:
improved performance of protein A chromatography increasing product purity
DNA and HCP levels in the protein A pool near regulation-required levels
reduction of turbidity-driven filtration requirements in downstream processing
protection of valuable protein A resin
size reduction of AEX polishing columns or more economical replacement of those columns with single-use membrane chromatography.
Dynamic Light-Scattering (DLS) Analysis: The effect on particle-size distribution in CCCF after clarification is one performance feature that differentiates the AEX chromatographic clarification capsules from conventional depth filters. DLS provides particle and dissolved polymer size-distribution information in the range of 0.8 nm to 3,500 nm. It is a useful technique that is more accurate and informative than turbidity measurement for characterizing the purity of primary harvest, centrate, and clarified fluids.
Because the intensity of light scattered by a particular particle scales with the sixth power of its size, DLS is conservative with respect to characterization of clarification performance. The technique is very sensitive to the largest particles present in a fluid; conversely, the smallest particles present may be obscured by the presence of relatively small numbers of larger particles. That is evident in the DLS particle size distribution plot for a CHO centrate fluid (Figure 1), which shows only particles in the range of 0.1–5.0 µm. Those large particles make it impossible to see small ones such as DNA and proteins that are also present in the centrate.
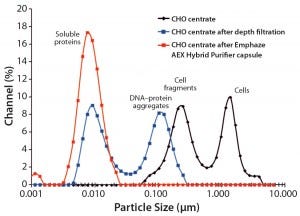
Figure 1: Size-distribution comparison of Chinese hamster ovary (CHO) centrate clarified either with a conventional depth filter or an anion- exchange (AEX) chromatographic clarification capsule; dynamic light- scattering data collected using a Nano-flex instrument from Microtrac, Inc.
Nevertheless, DLS measurement confirms the presence of particles with sizes that are consistent with cells and large cell fragments. After clarification with a fine-grade conventional depth filter, those large cell-debris components are effectively reduced, and the CCCF exhibits a bimodal distribution: one population of larger particles in the range of 50–300 nm and another population of smaller particles in the range of 5–30 nm. The larger particles indicate the presence of DNA/HCP aggregates and small cell debris, whereas the small particles indicate soluble centrate components such as proteins.
By contrast, centrate clarified using an AEX chromatographic clarification capsule exhibits a single distribution of particles in the range of 3–25 nm. That size distribution is consistent with the presence of mainly soluble particles such as proteins in the CCCF. By comparing the size distributions obtained using both devices, we found that using Emphaze AEX Hybrid Purifier capsule during clarification significantly reduces the load applied to a subsequent 0.2-µm sterilizing membrane. That minimizes the surface area needed and makes possible an economical transition to a 0.1-µm sterilizing membrane for superior protection of capture chromatography.
Turbidity Analysis: The type of size- distribution profile observed after using flow-through chromatography capsules results from removal of soluble contaminants such as DNA, HCP, and HCP– DNA aggregates during chromatographic clarification. Figure 2 shows similar throughputs (L/m2) during CHO centrate clarification for both the Emphaze AEX Hybrid Purifier capsule and a conventional fine-grade depth filter. Performance differences manifest themselves in turbidity and in reduction of DNA and HCPs.
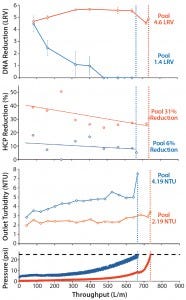
Figure 2: Performance comparison of a conventional fine-grade depth filter (blue) and an AEX chromatographic clarification capsule (orange) during CHO centrate clarification (initial turbidity 84 NTU); HCP reduction measured using a generic CHO host-cell protein (HCP) enzyme-linked immunosorbent assay (ELISA) from Cygnus Technologies; DNA reduction measured by quantitative polymerase chain reaction (qPCR) using a resDNASEQ quantitative CHO DNA kit from Life Technologies.
Turbidity reduction was nearly constant throughout the run with AEX chromatographic clarification capsules. By contrast, we observed a progressive turbidity increase for the depth filter as medium was loaded. As a result, pool turbidity after Emphaze AEX Hybrid Purifier flow-through was roughly half of that after depth filtration, indicating a cleaner solution.
More striking is the constant DNA reduction (>4 LRV) exhibited throughout operation of the AEX chromatographic clarification step. Depth filtration reduced DNA by >4 LRV in the first aliquot, but limited anion charge capacity led to a rapid breakthrough of DNA at roughly half the total throughput. Emphaze AEX Hybrid Purifier capsules provided a consistently higher HCP reduction throughout their run, yielding a 31% HCP reduction in the final pool compared with just 6% HCP reduction from a conventional depth filter.
Improving Capture Results
Implementing AEX chromatographic clarification increases the robustness of primary recovery from CHO cultures, improving control of the CCCF’s physical and biochemical characteristics. This robust primary recovery strategy can be platformed, scaled, and deployed more quickly than other options, with reduced risk of technical and/or regulatory failure. Enhanced reduction of turbidity, DNA, and HCP during primary recovery are proximate indicators of the value of implementing AEX chromatographic clarification upstream of protein A. But its true value to the overall DSP is evident when analyzing the effects of higher CCCF purity on protein A column performance.
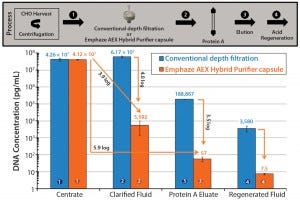
Figure 3: Comparing DNA clearance after protein A affinity chromatography (log scale) when using either a conventional fine-grade depth filter or an AEX chromatography clarification capsule during CHO centrate clarification; results were obtained using a column packed with MabSelect SuRe resin from GE Healthcare Life Sciences (in a 1.6 cm × 10 cm, 20-mL CV column loaded to 22 mg/mL IgG at 200 cm/h, washed with 5 CV PBS at pH 7.0, and eluted with 4 CV citrate buffer at pH 3.0). Acid regeneration was performed using 5 CV citrate buffer at pH 2.0. HCP reduction was measured with a generic CHO HCP ELISA from Cygnus Technologies, and DNA reduction was measured by qPCR using the resDNASEQ Quantitative CHO DNA kit from Life Technologies.
As Figure 3 shows, by removing >4 LRV of DNA during clarification, Emphaze AEX Hybrid Purifier capsules influence the performance of protein A columns not only by significantly increasing their DNA clearance, but also by decreasing the amount of DNA that remains bound to protein A resin after a capture cycle. DNA concentration in protein A eluate was 3.5 logs lower when AEX flow-through was used in clarification instead of a conventional depth filter. Also, DNA concentration in the acid-regeneration fluid was 2.7 logs lower for that clarified using AEX chromatography than for fluid clarified by depth filtration, which indicates substantially reduced column-bound DNA impurities after elution.
Using Emphaze AEX Hybrid Purifier capsules also has a profound positive influence on HCP clearance after protein A chromatography, as Figure 4 shows. HCP concentration in the protein A column eluate was 19 times lower after AEX Hybrid Purifier clarification than with that by conventional depth filtration. Protection of the Protein A column from resin-bound impurities was similarly enhanced by using AEX flow-through chromatography in the clarification stage, lowering HCP concentration in the clean- in-place (CIP) step by 17 times compared with using a conventional depth filter.
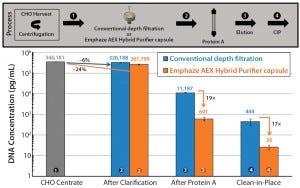
Figure 4: Comparing HCP clearance after protein A affinity chromatography (log scale) when using either a conventional fine-grade depth filter or an AEX chromatography clarification capsule during CHO centrate clarification; results were obtained using a HiScreen MabSelect SuRe column from GE Healthcare Life Sciences (0.77 cm × 10 cm, 4.7 mL CV column loaded to 22 mg/mL IgG at 200 cm/h, washed with 10 CV PBS at pH 7.0, and eluted with 10 CV 100 mM arginine buffer at pH 3.5). Cleaning in place (CIP) was performed using 4 M guanidine-HCl with 15 minutes contact time. HCP reduction was measured with a generic CHO HCP ELISA from Cygnus Technologies, and DNA reduction was measured by qPCR using the resDNASEQ Quantitative CHO DNA kit from Life Technologies.
The decreased levels of DNA and HCP that remain bound to the protein A column would suggest that alternative, less-aggressive cleaning strategies could be implemented to extend the life of that column and improve its cycle-to-cycle consistency. Even under the same cleaning conditions, using Emphaze AEX Hybrid Purifier capsules could extend the life of a protein A column by keeping more binding sites free of contaminants and delaying resin decay. An important ramification of our findings is that AEX flow-through chromatography improves the robustness of purification performance for the capture step, thus accelerating process development.
Mechanisms of Action
The higher product purity achieved when using AEX chromatographic clarification is in agreement with the referenced studies (10–14), suggesting that HCPs and chromatin-aggregate associations are the root causes of many impurities present in the protein A fraction. Research shows that which HCPs hitch-hike with a MAb product during protein A elution depends on the specific MAb itself.
Levy et al. identified several problematic HCPs using four different antibodies and observed that most HCPs associated with the product were acidic (11). Thus, it stands to reason that the AEX ligand present in Emphaze AEX Hybrid Purifier capsules will preferentially remove those acidic HCPs, making higher HCP clearance possible during protein A chromatography. Furthermore, by removing DNA, this technology also might be targeting chromatin catabolites that would otherwise bind to protein A resin, reducing its DBC and increasing impurity leaching leading to coelution.
DSP improvements in MAb operations are needed to keep pace with advances in upstream processing that have provided higher titers and cell culture densities. These downstream improvements should follow a holistic approach, wherein the integrated process and interactions among different unit operations are considered altogether rather than optimizing one unit operation at a time. Implementing AEX chromatography at the clarification stage significantly enhances the performance of protein A chromatography capture, easing the challenges faced by downstream polishing steps. Enhancing protein A performance translates into process simplification and increased robustness. A clarification strategy using Emphaze AEX Hybrid Purifier capsules can achieve higher product purity early in DSP, which brings great benefits to the entire manufacturing process and lowers operational costs for high-quality biopharmaceuticals.
References
1 Liu HF, et al. Recovery and Purification Process Development for Monoclonal Antibody Production. Landes Bioscience 2(5) 2010: 480–499.
2 Shukla AA, et al. Downstream Processing of Monoclonal Antibodies: Application of Platform Approaches. J. Chromatogr. B 848, 2007: 28–39.
3 Hogwood CE, Bracewell DG, Smales MC. Measurement and Control of Host Cell Proteins (HCPs) in CHO Cell Bioprocesses. Curr. Opin. Biotechnol. 30, 2014: 153–160.
4 Gottschalk U. Bioseparation in Antibody Manufacturing: The Good, the Bad and the Ugly. Biotechnol. Progr. 24(3) 2008: 496–503.
5 Farid SS. Process Economics of Industrial Monoclonal Antibody Manufacture. J. Chromatogr. B 848, 2007: 8–18.
6 Shukla AA, Hinckley P. Host Cell Protein Clearance During Protein A Chromatography: Development of an Improved Column Wash Step. Biotechnol. Progr. 24, 2008: 1115–1121.
7 Nogal B, Chhiba K, Emery J. Select Host Cell Proteins Coelute with Monoclonal Antibodies in Protein A Chromatography. Biotechnol. Progr. 28(2) 2012: 454–458.
8 Jin M, et al. Profiling Host Cell Proteins By Two-Dimensional Difference Gel Electrophoresis (2D- DIGE): Implications for Downstream Process Development. Biotechnol. Bioeng. 105(2) 2009: 306–316.
9 Hogwood CE, et al. The Dynamics of the CHO Host Cell Protein Profile During Clarification and Protein A Capture in a Platform Antibody Purification Process. Biotechnol. Bioeng. 110, 2013: 240–251.
10 Tarrant RDR, Velez-Suberbie ML, Tait A. Host Cell Protein Adsorption Characteristics During Protein A Chromatography. Biotechnol. Progr. 28(4) 2012: 1037–1044.
11 Levy NE, et al. Identification and Characterization of Host Cell Protein Product- Associated Impurities in Monoclonal Antibody Bioprocessing. Biotechnol. Bioeng. 111(5) 2014: 904–912.
12 Gagnon P, et al. Nonspecific Interactions of Chromatin with Immunoglobin G and Protein A, and Their Impact on Purification Performance. J. Chromatogr. A 1340, 2014: 68–78.
13 Gan HT, et al. Characterization and Removal of Aggregates Formed By Nonspecific Interaction of IgM Monoclonal Antibodies with Chromatin Catabolites During Cell Culture Production. J. Chromatogr. A 1291, 2013: 33–40.
14 Gagnon P, et al. Chromatin-Mediated Depression of Fractionation Performance on Electronegative Multimodal Chromatography Media, Its prevention, and Ramifications for Purification of Immunoglobin G. J. Chromatogr. A 1374, 2014: 145–155.
Corresponding author Angelines A. Castro- Forero is a senior product-development engineer, Zona Jokondo is a product-development biologist, Alexei Voloshin is global scientific services leader, and Jonathan F. Hester is a research specialist in life science process technologies at 3M Purification Inc., 3M Center, Building 236-1C-14, St. Paul, MN, 55144-1000; 1-651 733 0942, fax 1-651-733- 9525; [email protected], www.3M.com, www.3Mlifescience.com.
You May Also Like





