Standardizing Human MSCs As Critical Raw Materials in Cell Therapy Products: Streamlining Clinical Translation
WWW.ISTOCKPHOTO.COM
Advancements in cell therapy, biofabrication, and synthetic biology have driven the growth of the global regenerative medicine (RegenMed) industry in the past decade. The industry has developed innovative treatment options for patients with otherwise unmet medical needs (1). Human or animal cells or tissues are used as critical raw materials in cell therapy products that can replace, regenerate, or augment patients’ diseased, dysfunctional, or injured cells, tissues, or organs. These cells or tissues can be unmanipulated, or their biological characteristics can be altered ex vivo before administration of a final product to patients. Examples of such treatments range from traditional blood transfusions to recent approaches in autologous stem cell transplants to allogeneic engineered tissue substitutes (2).
The complexities associated with manufacturing these “living drugs” should not be underestimated. Each product comes with its own specific challenges and complexities for managing its development, manufacturing, distribution, and delivery. Such challenges are associated with securing a robust supply chain for starting materials, arranging related logistics, manufacturing, product release, therapy logistics, and treatment.
Human mesenchymal stem/stromal cells (hMSCs) are becoming a foundational technology for a variety of RegenMed products. These cells have been studied extensively over the past few decades, and hMSCs have found widespread adoption throughout RegenMed because of their broad spectrum of biological functions. They are multipotent progenitor cells with multilineage potential to differentiate into cell types of mesodermal origin, such as adipocytes, osteocytes, and chondrocytes. In addition, MSCs can migrate to sites of inflammation to exert potent immunosuppressive and antiinflammatory effects through interactions between lymphocytes associated with both innate and adaptive immune systems. And their ease of isolation and scalable ex vivo expansion lend a manufacturability that can be leveraged for widespread therapeutic commercialization.
hMSCs have been used or tested as critical raw materials in more than 900 global clinical trials for cell-based therapies, engineered tissues, or combination products. So they also face the above-mentioned challenges for clinical application and commercial uses. They have been tested in clinical trials for various indications, including orthopedic injuries, graft-versus-host disease (GvHD) following bone marrow transplantation, cardiovascular diseases, autoimmune diseases, and liver diseases (3). Furthermore, gene editing of MSCs to overexpress antitumor genes or therapeutic factors has provided new strategies over and above those that hMSCs could address inherently, thus greatly broadening their application.
MSC Applications and Future Demands
To date, hMSCs have been used to treat more than 36,000 patients globally, and more than 900 registered global clinical trials have used hMSCs (3). Several MSC products are approved in countries outside the United States. Regulatory authorities in Canada and New Zealand have granted marketing authorization to a product called Prochymal (Osiris Therapeutics) consisting of allogeneic bone-marrow–derived MSCs for treating steroid-resistant GvHD in children. Two MSC products are approved in South Korea: an allogeneic umbilical cord blood–derived MSC-based product (Cartistem) for treating degenerative arthritis and an autologous adipose tissue-derived MSC product (Cupistem) for treating anal fistulas in patients with Crohn’s disease. Other approved MSC-based products are Alofisel (TiGenix and Takeda, approved in Europe), Temcell (JCR pharmaceuticals, approved in Japan), Heartsheet (Terumo, approved in Japan), and HearticellgramAMI (FCB-Pharmicell, approved in South Korea). Efficacy data with a 70% response rate (paired with 180-day safety data released in February 2018) for MSC-100 IV (Mesoblast) treatment in a phase 3 GvHD trial may invite accelerated FDA approval. It has been suggested that a wave of new MSC-TPs (MSC therapeutic products) will gain FDA approval for sale by 2030, which would drive MSC demand to greater than 300 trillion cells per year (4).
A plethora of safety data in clinical trials and MSCs’ applicability toward treating various injuries and diseases have made them ideal candidates for the emerging biofabrication field. The application of MSCs in future engineered tissue and organ manufacturing will generate demand of an additional 300 trillion cells by 2040 (4). Extracellular vesicles (EVs) such as exosomes, have been implicated for a dominant mode of MSC action through paracrine signaling (5). That understanding has evolved because implanted cells often do not engraft or persist long term, but instead generate paracrine effects that are still observed after the cells have left the target site. Thus, MSC-EVs have strong potential as cell-free alternatives to MSC-therapy, but extensive bioprocessing developments for EV production technologies must be addressed before their future translation and clinical testing. Over the next 20 years, the estimated demand for MSC-EVs will create further demand for hMSCs in the same 300-trillion range as MSC-TPs (4).
Other than cell therapy applications, industries such as the cosmeceutical and food industries are actively engaging into research using hMSCs. L’Oreal and Johnson & Johnson are directing resources to add biological components, such as cytokines and growth factors from MSCs, to their personal care products and testing these products on bioprinted human skin models. The “clean meat” industry is trying to solve the upcoming global demand for meat innovatively by using MSCs as a raw material to generate engineered in vitro produced meat. Research is directed at using muscle precursor cells (satellite cells) and fat cells derived from MSCs for this purpose. A major current limitation is the high cost of generating enough cells at a commercially relevant scale, but as manufacturing advances there is a potential for a log reduction in cost over the next 20 years.
Quality Requirement for MSCs as Critical Raw Materials
Emerging technologies and new markets will cause a sharp rise in the demand for hMSCs for which products need to be consistent. Currently, high production costs associated with hMSC products hinder their widespread adoption, but new supply-chain innovations are coming to market to address this. The need for a source of high-volume CGMP-compliant hMSCs ready for “further manufacturing” will help developers of RegenMed products significantly reduce cost and time-to-market. Current good manufacturing practice (CGMP) is a system enforced by the US Food and Drug Administration to ensure that products are consistently sourced, manufactured, monitored, and tested according to specific quality standards. Global regulations for manufacturing raw materials for the cell therapy market are still under development. In the meantime, manufacturers of raw materials can provide GMP quality by following applicable GMP guidelines (e.g., 21 CFR 1271) recommended for pharmaceutical products. Those GMP guidelines provide guidance for manufacturing, testing, and quality assurance. When raw materials are manufactured according to all applicable GMP guidelines, documented evidence can be provided of purity, potency, consistency, and stability. GMPs therefore are one of the major quality assurance systems to ensure the safety and high quality of raw materials. To enable a seamless transition from preclinical development to the clinical stage, it is important to identify the appropriate GMP-grade raw materials and reliable suppliers during early stage preclinical research. Changing raw materials at a later stage in clinical development to meet the increased regulatory requirements often creates significant additional costs and lengthens the development timeline. This is driven primarily by the potential need to perform time-consuming clinical comparability studies. Switching to the required GMP-grade raw materials at an early stage (directly after preclinical development) will prevent the need for such studies and thereby bring a significant economic benefit.
Ancillary Materials: There will be a significant demand for high-productivity, CGMP bioprocess media as well to meet the future high demand for MSCs. Ancillary materials (AMs) — also known as ancillary products, ancillary reagents, and process reagents — fall under this ancillary material category. AMs are raw materials that are not intended to be present in a final product but are critically important in its manufacturing. Cell culture media is a significant AM for the manufacturing of MSCs. USP (United States Pharmacopeia) general chapter <1043> emphasizes the importance of proper AM selection and describes four risk-based categories for classifying critical materials. Tier 1 includes low-risk, highly qualified materials such as approved pharmaceutical products (e.g., pharmaceutical-grade heparin). Tier 2 AMs are low-risk, well-characterized materials intended for use as AMs and produced in compliance with GMPs. Tier 3 includes materials with moderate risk, often not intended for use as AMs (e.g., reagent-grade materials used in other applications or industries). High-risk materials are categorized as Tier 4. They are not produced in compliance with CGMPs or intended to be used in cell therapy manufacturing. Based on these categories, companies can devise qualification or risk-reduction activities for their AMs.
Three core quality requirements for MSCs and bioprocess media are intended for further manufacturing based on phase appropriateness.
Raw materials must be safe. The origin and impurity profile of all materials used for manufacturing of GMP-grade raw materials should be assessed, including an assessment of all materials for the presence of animal-derived components in their respective manufacturing. The industry is moving away from using animal-derived components in the manufacturing processes for regulatory/safety reasons.
Raw materials should be manufactured following applicable GMP guidelines to provide documented evidence of purity, potency, consistency and stability. These include, but are not limited to the following:
Manufacturing and quality control (QC) according to standard operating procedures (SOPs)
Qualified and trained personnel
High-class cleanroom facility and qualified equipment
Validated and consistent processes (manufacturing, cleaning, QC methods)
Monitoring of product quality by in-process controls (IPC) at appropriate manufacturing steps
Product release according to predefined specifications by a quality unit completely independent from manufacturing.
Raw materials must comply with regulatory requirements.
USP <1043>, USP <92>, and Ph. Eur. general chapter 5.2.12
Manufacturing in compliance with relevant GMP guidelines, including key GMP demands such as change control and out-of-specification (OOS) procedures
Analytical methods based upon relevant International Council for Harmonisation (ICH) quality guidelines.
Key Technologies for Cell Therapy Product Development
RoosterBio Inc. is a human stem cell manufacturing company focused on accelerating commercialization of hMSC-based RegenMed products by providing standardized product platforms that enable rapid production and process development for streamlined clinical translation.
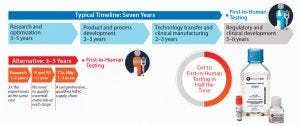
Figure 1: Standardized hMSCs and paired bioprocess media systems can significantly shorten cost and time to market.
MSCs have graduated from being a research tool to the level of a technology — similar to transistors or microchips. Like microchips, they are complicated technologies that need to be manufactured with stringent quality specifications. An electronics developer does not have to know how to build transistors and assemble a microchip but can buy microchips off the shelf and then design a product using them. To simplify the process of initiating clinical trials and reduce the time required to develop and characterize these critical raw materials, RoosterBio has developed high-productivity media and off-the-shelf CGMP-grade hMSC working cell banks as raw materials for further manufacturing. The CliniControl brand offers bioprocess media for further manufacturing and building a working cell bank. The platform enables product developers to reduce significantly the costs and time-to-market for their regenerative medicine products (Figure 1). The brand is supported by robust performance and characterization data as well as a complete safety testing panel and FDA master files for cross-reference. Cell and gene therapy manufacturers can integrate this off-the-shelf “microchip” of the RegenMed industry into their products to significantly reduce qualification and validation efforts. Suppliers of critical raw materials can help streamline the journey of their end users through the chemistry, manufacturing, and controls (CMC) submission process. Toward being the industry standard, RoosterBio, offers the following support on working cell banks and bioprocess media “for further manufacturing” uses:
Auditing of the production site
Providing cell and media master files for cross-reference
Providing regulatory support files and transmissible spongiform encephalopathy (TSE) certificates
Sending clients change notifications in advance of relevant changes
Providing additional product data on request (e.g., a QC brief)
Customizing support to meet special compliance needs for quality agreements and services to facilitate products into a range of manufacturing strategies.
Meeting the Needs for Critical Raw Materials
Recent breakthroughs in developments in manufacturing and engineering of MSCs have made them ideal sources for future regenerative medicine products. These adaptable cells can serve as versatile tools for disease treatment, even though the full understanding of their mechanisms is still developing. After hundreds of preclinical and human clinical trials using MSCs, they are now on a path for commercialization, and many trials have been accomplished (6). During the past decade, many experimental and clinical assays had been developed; however, several questions related to MSCs biology are unsolved. Future MSC-related research should identify more suitable markers to isolate source-specific MSCs and explore a basic understanding of growth regulators in differentiation and transdifferentiation and site-specific homing that can revolutionize cell regeneration therapies. hMSCs are a raw material for RegenMed applications, requiring dedicated efforts from manufacturing sciences to deliver high-quality, well-characterized, and functional MSCs toward creating a robust supply chain for meeting current and future MSC demands. New technologies in manufacturing sciences will reduce the cost of goods, enabling widespread adoption of hMSC technologies into RegenMed products. Standardizing hMSCs as critical raw materials will streamline clinical and commercial translation, effectively revolutionizing product development for the medical products of tomorrow.
References
1 Mount NM, et al. Cell-Based Therapy Technology Classifications and Translational Challenges. Philos. Trans. R. Soc. Lond. B Biol. Sci. 370(1680): 20150017; doi:10.1098/rstb.2015.0017.
2 Mahla RS. Stem Cells Applications in Regenerative Medicine and Disease Therapeutics. Int. J. Cell Biol. 2016; 2016: 6940283; doi:10.1155/2016/6940283.
3 Cell Trials data; www.celltrials.org.
4 Olsen TR, et al. Peak MSC — Are We There Yet? Front. Med. 5 (2018): 178; doi:10.3389/fmed.2018.00178.
5 Rani S, et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol. Ther. 23(5) 2015: 812–823; doi:10.1038/mt.2015.44.
6 US National Institutes of Health (database); https://clinicaltrials.gov.
Corresponding author Mithu Majumder, PhD, MBA, PMP, is senior project manager, Operations; Tim Olsen, PhD, is account manager; and Jon Rowley, PhD, is founder and CTO at RoosterBio, 5295 WestView Drive, Frederick, MD 21703, USA; [email protected].
You May Also Like





