Regenerative Medicine for Cardiovascular Disease: Characterization and Clinical Success
April 10, 2024
Cardiovascular diseases represent the leading cause of death globally, with nearly 18 million fatalities occurring each year (1). Pharmacological interventions mainly address symptoms rather than root causes of such diseases. Unfortunately, many patients do not respond adequately to treatment, so their conditions progress to congestive heart failure, eventually necessitating heart transplant. Organ supplies are limited, however, with a waiting list that continues to grow each year. Complications also can arise from immunosuppressive drugs that patients must take indefinitely after receiving donor hearts, significantly limiting applications of the transplant option.
Cell-based therapies have emerged as a promising alternative because they could regenerate heart tissue. Results from several clinical studies — based on different experimental designs and involving more than 3,000 patients in total — confirm the feasibility and safety of such procedures. Conducting one of those studies was Philippe Hénon, the founder of CellProthera, a French clinical-stage biotechnology company that develops cell-based therapies for ischemic diseases. In a pilot clinical study, Hénon demonstrated the feasibility, safety, and long-term efficacy of intramyocardial injection of cluster of differentiation 34 (CD34)–positive stem cells in patients who experienced acute myocardial infarction (AMI) (2). Patients received the therapy months within infarct, except for one trial subject who received it eight years later.
The latter patient did not respond to treatment because a lesion already had reached an advanced stage of fibrosis and calcification by the time of treatment. However, all of the other patients in the study experienced improvements in left ventricular ejection fraction and scored better New York Heart Association (NYHA) grade values several months after transplantation. Those patients also exhibited myocardial-structure regeneration and revascularization with recovery of contractility in previously akinetic areas, as demonstrated by positron emission tomography (PET). Apart from the patient who received therapy too late in disease progression, trial subjects survived for an average of 17 years (3). That outcome represents a significant improvement compared with the 50% mortality rate that clinicians generally observe five years after onset of AMI. Clinical benefit even was extended to three patients who were recommended initially for heart transplantation; several years after receiving CD34+ cell therapy, they no longer needed transplants.
Such therapies are effective because they harness bodies’ self-repair mechanisms. After AMI, damaged tissue secretes a complex blend of cardioactive chemokines that recruits and stimulates CD34+ cells, which then travel from bone marrow to peripheral blood. Post-AMI peripheral-blood concentrations of CD34+ cells correlate significantly with heart regeneration and functional improvement (4). CD34+ cells promote cardiac repair by releasing soluble paracrine factors and exosomes containing microRNA (miRNA) molecules that induce angiogenesis and revascularization of damaged tissue. Those paracrine factors also enhance proliferation of resident cardiomyocytes, reducing fibrosis and attenuating remodeling effects. Scar-associated chemokines attract CD34+ cells to the ischemic zone and induce their commitment into endothelial pathways.
CellProthera intends to maximize the therapeutic potential of that natural repair mechanism by harvesting CD34+ cells from a patient’s peripheral blood, expanding them in culture, and ultimately readministering them to the patient. The company has developed an automated, closed platform — called the StemXpand system — for good manufacturing practice (GMP)–compliant expansion of such cells. The system has received ISO 13485:2016 certification, and in June 2013, the European Medicines Agency (EMA) registered ProtheraCytes stem-cell preparations as an advanced-therapy medicinal product (ATMP) (EMA/370710/2013). Expansion also leverages StemPack single-use cell-culture kits and StemFeed medium, which is optimized for CD34+ cells (see the “Novel Platform” box).
Product Characterization
During in vitro characterization of their growth-factor secretion, exosome secretion, gene expression, cell surface marker expression, differentiation potential, and angiogenic potential, all clinical batches of ProtheraCytes products showed consistent secretion of vascular endothelial growth factor (VEGF), which is critical to angiogenesis (5). As Figure 1 shows, VEGF concentrations in the supernatants of ProtheraCytes cultures also correlated significantly with postexpansion numbers of CD34+ cells. Panel A depicts VEGF concentrations in samples from four healthy donors and 16 AMI patients after nine days of CD34+ cell expansion. Scientists observed no significant differences in VEGF concentrations between patient and healthy-donor samples, but significant differences were observed between patient samples and StemFeed media preparations (p-value = 0.0007) and between healthy-donor samples and StemFeed preparations (p = 0.0087) (Panel B). When normalizing VEGF concentrations and CD34+ cell counts, scientists observed no significant difference in the amount of factor secreted per cell in healthy donors (4.1 fg/cell) and AMI patients (4.4 fg/cell) (p = 0.6343) (Panel C). Panel D plots VEGF concentration and postexpansion CD34+ cell counts, and Panel E shows significant correlation between postexpansion VEGF concentration and CD34+ counts (Pearson correlation coefficient = 0.7484, p = 0.0009).
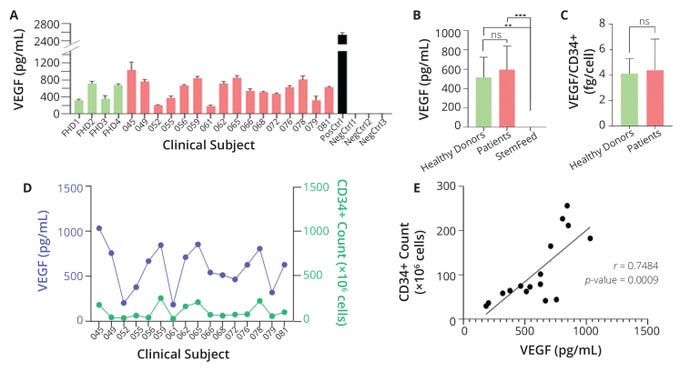
Figure 1: Quantification of CD34+ cells and vascular endothelial growth factor (VEGF) in the supernatant of ProtheraCytes cultures; panel (A) depicts VEGF concentrations in samples from healthy donors (green bars) and patients with acute myocardial infarction (AMI, red bars) after nine days of expansion. The black bar represents a positive control (PosCtrl), and three negative controls (NegCtrls) showed no VEGF levels. Panel (B) compares normalized VEGF concentrations among patient, healthy-donor, and StemFeedmedia samples. Panel (C) shows the average amount of VEGF secreted per CD34+ cell in healthy-donor and patient samples. Factor concentrations and cell counts are shown in panel (D), and panel (E) plots their relation. Asterisks indicate levels of increasing statistical significance (scale of one to four stars); ns denotes a result without statistical significance. (FIGURE ADAPTED FROM ARIES ET AL., 5)
Such results enabled development of a potency assay based on VEGF quantification. The assay leverages a Bio-Techne ELLA automated platform for enzyme-linked immunosorbent assays (ELISAs), which helps to minimize errors, reduce analysis time, and increase reproducibility. The method’s consistency, reliability, and ease of use enable quick release of clinical batches (4). The secretomes of expanded CD34+ cells also exhibited angiogenic activity during tube-formation assays (data not shown).
Subsequent study of the cells’ secretomes focused on analysis of extracellular vesicles (EVs). Size-distribution and concentration measurements of ProtheraCytes-derived exosomes were performed using a NanoSight NS300 analyzer from Malvern Panalytical and the exosome membrane markers were quantified by flow cytometry using the MACSPlex Exosome kit (Miltenyi Biotec). Each expanded cell secretes ~16,000 EVs with an average size of 86.7 ± 10.17 nm. Tested EVs expressed the characteristic exosomal markers CD63, CD81, and CD9, as well as endothelial markers (CD49e and CD44) and stem-cell markers (CD133) (5). The EVs also contained miRNAs with proangiogenic (130a, 126, 378, and 26a), antiapoptotic (21 and 146a), antifibrotic (133a, 24, 29b, and 132), and myocardial-regeneration activity (199a and 590).
Figure 2 depicts results from characterization of RNA content secreted by expanded CD34+ cells and their EVs. Samples from seven AMI patients were collected, subjected to the ProtheraCytes process, and analyzed by real-time polymerase chain reaction (RT-PCR). Then, data were normalized relative to let-7a miRNA levels. As determined by a Mann–Whitney test, exosome secretion of miRNAs was significantly higher than it was for the cells (Figure 2).
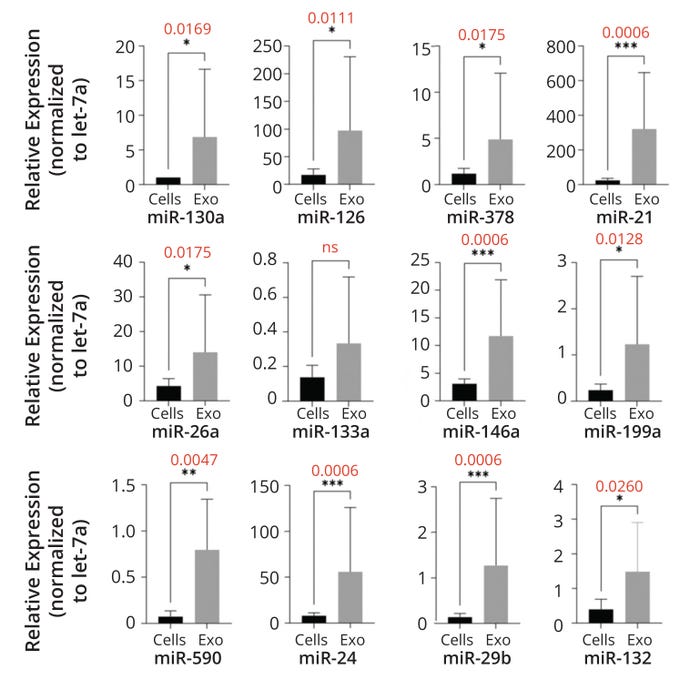
Figure 2: Exosomes derived from ProtheraCytes preparations contain proangiogenic, antiapoptotic, antifibrosis, and cardiac microRNA (miRNA) molecules. The graphs below compare relative cellular (Cells) and exosomal (Exo) expression of given miRNAs. Values in red are p-scores. Asterisks indicate levels of statistical significance (scale of one to four stars); ns denotes a result without statistical significance. (FIGURE ADAPTED FROM ARIES ET AL., 5)
RNA sequencing (RNAseq) of clinical batches demonstrated that cells in ProtheraCytes preparations express endothelial genes and genes involved in angiogenesis and vasculogenesis (5).
Flow cytometry (FC) analysis of cell surface markers also indicated that the therapeutics express stem-cell markers (CD184, CD44, CD133, and CD117) and endothelial markers (CD31 and CD49b). It is a well-established fact that CD34+ cells contain endothelial progenitor cells (6). Cells in ProtheraCytes preparations likewise can differentiate into endothelial cells in vitro. During a 17-day differentiation protocol, CD34+ cells undergo clear morphological changes that are characteristic of endothelial cells, become adherent, and express endothelial markers: von Willebrand factor (vWF), CD31, VEGF receptor 2 (VEGFR-2), and vascular cell adhesion molecule 1 (VCAM-1) (5). Human CD34+ cells also contribute to microvessel formation when transplanted into ischemic rodent models (7).
A Novel Platform for Expansion of Autologous CD34+ Cells CellProthera has developed an automated platform that enables standardized and reproducible production of ProtheraCytes autologous CD34+ cell preparations, which have been authorized as an advancedtherapy medicinal product (ATMP) by the European Medicines Agency (EMA), France’s Agence Nationale de Sécurité du Médicament et Des Produits de Santé (ANSM), and the United Kingdom’s Medicines and Healthcare products Regulatory Agency (MHRA). The cellexpansion platform comprises a StemXpand automated device and StemPack single-use cell-culture production kits. The technology clinically validated for good manufacturing practice (GMP) production of clinical-grade cell therapies, and it has shown consistently high quality across multiple production sites. The system is patent-protected globally and ISO-13485:2016–certified. Clinicians will use the platform in US and EU trial centers during a phase 3 study evaluating the safety and effectiveness of ProtheraCytes preparations in patients who have experienced acute myocardial infarction (AMI). The platform and ProtheraCytes candidate also will be applied in clinical studies for ischemic- and renal-disease at the Shonan Kamakura General Hospital (SKGH) in Japan through a collaboration established with stem-cell pioneer Takayuki Asahara, MD, PhD (professor of physiology at the Tokai University School of Medicine). |
Safety and Efficacy Studies
Preclinical studies in AMI rodent models have shown that intramyocardial injection of ProtheraCytes preparations can improve cardiac function and regenerate heart tissue (8). Animals that received the transplanted cells exhibited significantly higher cardiac output and thicker ventricle walls compared with control animals. The therapy also proved to be safe, with no signs of toxicity, tumor formation, or cell biodistribution outside the subjects’ hearts.
After the successful results of the preclinical and pilot clinical studies, CellProthera started a multicenter, randomized, controlled phase 1–2b trial in severe AMI patients across multiple clinical sites in France and the United Kingdom (NCT02669810). Enrollment concluded in September 2023, with
33 patients in the treatment group receiving intramyocardial injections of the therapy and 16 patients in the standard of care (SoC) control group. The primary endpoint of the study is to evaluate the safety of ProtheraCytes preparations at a six-month follow-up. The secondary endpoint is to establish the candidate’s efficacy profile at a six-month follow-up by evaluating several surrogate markers by cardiac magnetic resonance imaging (cMRI). In this study, patients were selected eight to 10 days after infarction to exclude people who might have recovered naturally. Only patients with akinetic segments are selected so that the regeneration effect can be studied. Analysis of the clinical data is ongoing, and final results will be published in 2024.
The therapy’s success stems from four key factors: timing of injection, cell type, cell number, and injection route (9). Administration should occur within two to three months of myocardial infarction to maximize cell engraftment. Previous cell therapies based on similar approaches used bone-marrow–derived mononuclear cells, of which only 0.5–1% are CD34+. By injecting CD34+ cells that promote angiogenesis, chances increase for revascularization and regeneration in ischemic lesions. High doses of cells should be administered because clinical studies have shown that effects are dose dependent. And of course, route of administration is critically important. Intramyocardial injection results in the highest cell retention when compared with other routes (10).
New Possibilities for Treating Ischemic Conditions
Therapies based on CD34+ cells show promise for treating ischemic stroke, which is the second leading cause of death worldwide, accounting for 5.5 million deaths every year. The condition is a common cerebrovascular disease that results in high rates of disability and exacts over US$100 billion worth of associated costs. The disease has three phases: Its acute phase occurs within one week of stroke onset. A subacute phase occurs between one week and six months after a stroke. And patients enter a chronic phase after six months. Current treatment strategies include thrombolysis and mechanical thrombectomy, but those are limited by narrow windows for application: 3–4.5 hours and 24 hours, respectively. Thus, only 5–10% of eligible patients receive such treatments.
CellProthera’s approach to treating ischemic stroke also is based on CD34-directed repair mechanisms. After ischemic stroke, CD34+ cells are mobilized from the bone marrow to the peripheral blood. Concentrations of CD34+ cells in poststroke peripheral blood correlate significantly with neurological and functional improvement (11). Such cells release soluble paracrine factors and exosomes containing miRNAs that induce angiogenesis and revascularization of damaged brain tissue. CD34+ cells also move to the ischemic zone, differentiate into endothelial cells, and ultimately promote reestablishment of microvasculature. Harvesting, expanding, and administering patient CD34+ cells will help to maximize that natural repair mechanism.
With Cesar Borlongan, an expert in the ischemic stroke and director of the Center for Excellence for Aging and Brain Repair at the University of South Florida, CellProthera has studied the safety and effectiveness of ProtheraCytes therapy in the established middle cerebral artery occlusion (MCAO) rat model. The team tested three routes of administration: intraarterial (IA), intracerebral (IC), and intranasal (IN). On day 0, animals underwent MCAO surgery and behavioral testing. Then, on day 3, either ProtheraCytes preparations or vehicle media only were administered via IA, IC, or IN routes. Additional behavioral testing occurred on days 7, 14, and 28. On day 28, the animals were sacrificed for histological analysis (12).
Compared with control animals that received vehicle media only, test subjects that received ProtheraCytes cells by any route of administration showed marked improvements in elevated body swing, forelimb akinesia, paw grasp, and beam walk tests (12). Some animals treated with the therapy even returned to baseline values. Meanwhile, MCAO animals transplanted with the CD34+ cells by any route of administration experienced significant reductions in infarct area and cell loss compared with what was observed in control animals (Figure 3). Researchers observed reduced inflammation and evidence of human CD34+ cell engraftment in the transplanted animals. Those subjects also exhibited increased neurogenesis, angiogenesis, and EV expression, supporting the hypothesized mechanism of action (MoA) for ProtheraCytes therapy. In an in vitro stroke model, ProtheraCytes preparations showed protective effects and promoted the survival and metabolic activity of primary neurons exposed to oxygen glucose deprivation (12). Overall, such results indicate that autologous CD34+ cell therapy has angiogenic and regenerative properties that could be used to treat conditions such as critical limb ischemia and traumatic brain injury.
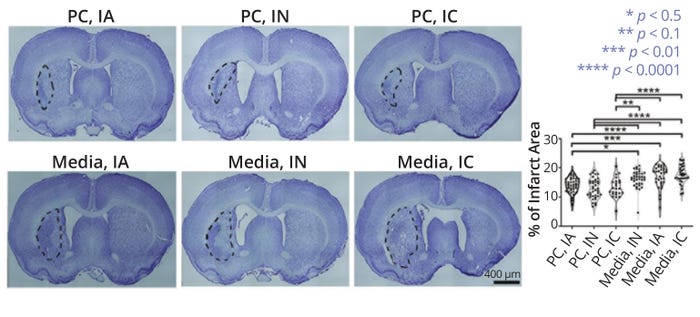
Figure 3: Nissl staining of coronal brain sections from middle cerebral artery occlusion (MCAO) models, with infarct areas outlined in black; the top images show subjects that received ProtheraCytes preparations (PC) via intraarterial (IA), intranasal (IN), and intracerebral (IC) administration. The bottom images are from subjects that received only therapy vehicle media. Subjects that received CD34+ therapy showed significantly smaller infarcts than did nontreated models. (FIGURE ADAPTED FROM LEE ET AL., 12)
References
1 Cardiovascular Diseases (CVDs). World Health Organization: Geneva, Switzerland, 2021; https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds).
2 Pasquet S, et al. Long-Term Benefit ofIntracardiac Delivery of Autologous Granulocyte Colony-Stimulating Factor-Mobilized Blood CD34+ Cells Containing Cardiac Progenitors on Regional Heart Structure and Function After Myocardial Infarct. Cytotherapy 11(8) 2009: 1002–1015; https://doi.org/10.3109/14653240903164963.
3 Hénon P, Bischoff N, Dallemand R. Transforming Perspectives in Cardiac Cell Therapy: Hypothesis and Commentary Following Updated Results of a Pilot Study Investigating Very Long-Term Clinical Outcomes in Severe AMI Patients Following Trans-Epicardial Injection of Peripheral Blood CD34+ Cells. Stem Cell Rev. Rep. 20, 2024: 247–257; https://doi.org/10.1007/s12015-023-10643-w.
4 Leone AM, et al. Mobilization of Bone Marrow-Derived Stem Cells After Myocardial Infarction and Left Ventricular Function. Eur. Heart. J. 26(12) 2005: 1196–1204; https://doi.org/10.1093/eurheartj/ehi164.
5 Aries A, et al. Development of a Potency Assay for CD34+ Cell-Based Therapy. Sci. Rep. 13(19665) 2023; https://doi.org/10.1038/s41598-023-47079-8.
6 Asahara T, et al. Isolation of Putative Progenitor Endothelial Cells for Angiogenesis. Science 275(5302) 1997: 964–966; https://doi.org/10.1126/science.275.5302.964.
7 Park S-W, et al. Efficient Differentiation of Human Pluripotent Stem Cells into Functional CD34+ Progenitor Cells by Combined Modulation of the MEK/ERK and BMP4 Signaling Pathways. Blood 116(25) 2010: 5762–5772; https://doi.org/10.1182/blood-2010-04-280719.
8 Saucourt C, et al. Design and Validation of an Automated Process for the Expansion of Peripheral Blood-Derived CD34+ Cells for Clinical Use After Myocardial Infarction. Stem Cells Translat. Med. 8(8) 2019: 822–832; https://doi.org/10.1002/sctm.17-0277.
9 Hénon P. Key Success Factors for Regenerative Medicine in Acquired Heart Diseases. Stem Cell Rev. Rep. 16, 2020: 441–458; https://doi.org/10.1007/s12015-020-09961-0.
10 Vrtovec B, et al. Comparison of Transendocardial and Intracoronary CD34+ Cell Transplantation in Patients with Nonischemic Dilated Cardiomyopathy. Circulation 128(11s1) 2013: s42–s49; https://doi.org/10.1161/circulationaha.112.000230.
11 Dunac A, et al. Neurological and Functional Recovery in Human Stroke Are Associated with Peripheral Blood CD34+ Cell Mobilization. J. Neurol. 254(3) 2007: 327–332; https://doi.org/10.1007/s00415-006-0362-1.
12 Lee J-Y, et al. Probing Multiple Transplant Delivery Routes of CD34+ Stem Cells for Promoting Behavioral and Histological Benefits in Experimental Ischemic Stroke. Stem Cells Translat. Med. 13(2) 2024: 177–190; https://doi.org/10.1093/stcltm/szad081.
Ibon Garitaonandia is chief scientific officer of CellProthera, 12 Rue du Parc, 68100 Mulhouse, France; 33-3-69-71-97-71. Please send inquiries to Suzanna Muggleton at [email protected].
You May Also Like





