Welcome to the 2015 BioProcess Theater and Zone!
May 12, 2015
 Not too long ago, many bioprocessing professionals perceived the annual BIO International Convention as an event about biotechnology business, financial matters, investing, and partnering — and until not too long ago, for the most part, that was correct.
Not too long ago, many bioprocessing professionals perceived the annual BIO International Convention as an event about biotechnology business, financial matters, investing, and partnering — and until not too long ago, for the most part, that was correct.
In 2007, the Biotechnology Industry Organization and BioProcess International formed a partnership to create a dedicated destination where bioprocessing professionals could learn about the latest technologies and trends in biopharmaceutical development and manufacturing. Thus was born the BioProcess Theater and Zone.
Every year since then, leading biopharmaceutical suppliers have exhibited in the BioProcess Zone to showcase their experience, innovations, and services to a BIO audience. The BPI Theater is a dedicated amphitheater located on the exhibition floor at the heart of the BioProcess Zone (Figure 1). It is designed to supplement BIO’s main educational program and provide convention attendees with complementary scientific programming every day — while also serving as a unique destination for biopharmaceutical professionals to meet, learn, and network.
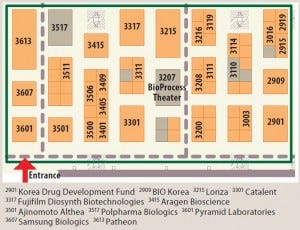
Figure 1: This map of the BioProcess Zone in the exhibition hall at the 2015 BIO International Convention, with the largest booths identified as
landmarks, shows the location of the BioProcess Theater. Search exhibitors online
This official guide to the 2015 BioProcess Theater and Zone provides you with an exclusive preview of this year’s theme: “the resurgence of the third-party partnership.” It will include four outsourcing-focused tracks with 20 scientific presentations and two roundtable events.
As this guide illustrates, topics covered will fall under four general categories:
analytical services
cell line and product/process development
formulation, fill and finish
Clinical and commercial manufacturing.
On Tuesday and Wednesday at lunchtime, roundtable discussions will cover
innovation strategies for speeding development, lowering costs, and enhancing quality
managing the contract relationship.
Super (Bioprocess) Heroes: It is your tireless work ethic, imagination, and enduring vision that have enabled the biopharmaceutical industry to undergo the dramatic transformations described in this supplement. You have made incredible technological advances and essentially rewritten the book on how to develop and manufacture biotherapeutics more effectively and less expensively while delivering them to a global patient base. Because of you, physicians and clinicians around the world can better treat illnesses, prevent disease, and improve quality and length of life for their patients.
As the premier scientific publication to the biopharmaceutical industry, BioProcess International takes its responsibility seriously: to provide a forum where the “superheroes” of bioprocessing can come together, to publish and to present you with the most pertinent information necessary to tackle today’s process challenges and improve future bioprocesses.
We hope you enjoy this guide and find it useful. Come visit us at the BioProcess Theater and the BioProcess Zone in Philadelphia this June.
Best regards,

Brian Caine
Cofounder and Publisher
BioProcess International
Roundtables on Thursday 18 May 201510:30 am – 12:00 pm 1:00–2:30 pm More information on the 2015 BIO Convention: http://convention.bio.org |
From the OrganizersThe world’s largest, most influential biotech meeting is coming to the Pennsylvania Convention Center on 15–18 June 2015, and you won’t want to miss it. The BIO International Convention regularly attracts 15,000 of the most powerful biotech and pharma players from 65 countries, offering powerful business partnering, networking, and education. At this year’s Convention, we will be honoring the everyday superheroes of biotechnology. These men and women may not always make headlines, but every day they put in the hard work needed to move our industry forward in the laboratory, Enjoy Philadelphia, and have an enjoyable and productive convention! |
You May Also Like





