An Easy Method for Increasing Cell Yields of Insect Cells in a Benchtop Bioreactor

Figure 1.
This study describes a simple procedure for improving insect cell yields in a benchtop cell culture bioreactor. Here, yields of Spodoptera frugiperda (Sf-9) cells were increased by nearly 29% through monitoring dissolved carbon dioxide (dCO2) levels in the culture and adding air to the vessel headspace to reduce dCO2. The method can also be used to maximize yields in a wide range of mammalian cell types.
Introduction
Producing high yields of protein from insect cells usually requires maintaining high levels of dissolved oxygen (DO) in the culture. However, as cell concentrations grow, they generate an ever-increasing level of dCO2′ which can inhibit cell growth. We compared growth of Sf-9 insect cell yields in two runs, first without control of dCO2 levels and then by continuously flowing air into the vessel headspace above the liquid media level to reduce dCO2 concentration. This insect cell culture protocol has not been fully optimized to obtain the highest yields possible — but it is meant to serve as a guide for basic procedures and materials.
Materials and Methods
The Bioreactor: We used a 2.5-L total volume New Brunswick Scientific (NBS) CelliGen® 310 benchtop autoclavable bioreactor with a pitched-blade impeller (NBS Catalog Number M1287-1260). The CelliGen 310 is a CGMP-compliant system designed for high-density growth of mammalian, plant, and insect cell lines. It comes standard with a large 15-in. Industrial color touchscreen interface with an advanced controller to simplify setup, calibration, and control. The bioreactor includes three built-in pumps, pH/DO and level/foam probes, and one thermal mass flow controller (TMFC) for regulating gas flow. The CelliGen 310 used in this study had a TMFC range of 0.1-5 L/min (other flow ranges or choice of multiple TMFCs are also available).
We added three optional accessories. A Mettler Toledo dCO2 sensor and transmitter (Catalog Nos. P0720-6480 and P0620-6580) were connected to the CelliGen 310 to measure dCO2 concentration throughout the process. An NBS accessory gas overlay controller (No. M1287-3550) with a flow range of 0.1-5 L/min (capable of regulating four gases) was used to regulate addition of air to the vessel headspace. And an optional NBS BioCommand® Plus supervisory software package (No. M1291-0000) was also used to automatically log data. Additonally, a gas overlay vessel kit that includes neccessary tubes, filters, and fittings (No. M1287-3505) is highly recommended.

The benchtop CelliGen 310 bioreactor is a versatile research tool for optimizing cell growth and production of mammalian, insect, and plant cell lines. Multiple connections, easily accessible from the side and rear, provide ability to integrate data from all your ancillary devices, such as dCO2 sensors, a gas overlay controller, and SCADA software. A large touchscreen interface makes it easy to set up, calibrate, and monitor each run, as well as export data and trend graphs to a PC. ()
Overview: (1) Autoclave the vessel with phosphate buffer solution (PBS) for 60 minutes. (2) Remove PBS from the vessel. (3) Add 1.5 L of insect cell media to the vessel. (4) Inoculate 500 mL of insect cell suspension with a starting cell count of 4.0 × 105 cells/mL.
Medium: We used Sf-900 II serum-free media (No. 10902-088).
Inoculum: A proprietary Sf-9 cell line was supplied by a leading biotechnology company. The inoculum was cultivated in a NBS open-air innova® 2000 shaker placed inside an incubator for temperature regulation.
Control Setpoints
The following setpoints were keyed into the touchscreen controller prior to inoculation:
temperature 28°C
DO 40%
agitation 70 rpm, gradually increased to 100 rpm over the course of the run.
DO Control: The DO probe provided as part of the CelliGen 310 kit was calibrated at 0% (obtained by briefly disconnecting the cable), then calibrated at 100% (obtained using 100 rpm agitation and 5 L/m airflow rate). The control was set to “fourgas” mode to automatically maintain the DO setpoint by sparging three gases (air, O2 and N2).
pH Control: Although pH control capability is built into the CelliGen 310 bioreactor, it is not generally needed for controlling insect cell growth and was therefore not regulated in this study.
Fed-Batch Control: Pumps were calibrated using standard-supplied tubing to keep track of liquid quantities entering and exiting the vessel. Samples were taken several times a day (as described below) to measure glucose and cell density.
Pump Calibration: To assure the most accurate flow rate, calibrate the pump each time you change tubing. (1) Use about two feet of tubing for each line attached to a pump head. (2)Insert tubing into the appropriate pump head. (3) Set the pump assignment to “None.” (4) Select “Calibrate” and either “15”, “30,” or “60” seconds as the time interval. Record the quantity of water pumped into a graduated cylinder for the defined time period, and enter that into the “Flow Rate” field, then hit “OK.” Your flow rate will be automatically calculated.
Control Program: For these studies, we used one of three built-in pumps. When cells reached a density of 6 × 106 cells/mL, 20 ml/L of Yeastolate (Invitrogen Catalog No. 18200-048) was automatically added by Pump #1.
In both runs, DO was sparged into the liquid media and controlled by the CelliGen 310’s built-in four-gas controller. In run 1, we added no gas overlay into the vessel headspace. In the second run, all conditions remained the same, but 300 mL/min of air was continuously added to the vessel headspace to reduce dCO2 concentration (control screen shown in Figure 1).

Figure 1. ()
Results and Discussion
As shown in Figure 2, maximum viable cell density in run 1 reached 9.18 × 106 cells/mLon day 9. In run 2, maximum cell density increased by 28.5%, reaching 11.8×106 cells/mL on day 6. This study shows that by reducing dCO2 concentration, maximum cell density not only was significantly improved, but was also achieved at a faster growth rate.
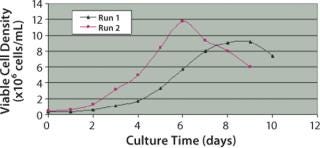
Figure 2. ()
It should be noted that the CelliGen 310 bioreactor can simultaneously control more than 120 process loops (32 loops per vessel, four vessels simultaneously), making it an extremely powerful research tool. It can be operated in batch, fed-batch, or perfusion modes and comes with choice of four interchangeable vessels (2.5-14.0 L) as well as a wide range of specialized impellers to maximize yields. For secreted products, a patented packed-bed basket option is available to maximize cell productivity regardless of cell type. The system includes multiple analog inputs/outputs for easily integrating data from up to 10 ancillary devices, such as additional TMFCs, sensors, scales, or on-line gas and glucose analyzers for optimized process control. However, we did not take advantage of the full potential of the 310, intending only to provide an easy technique for increasing yields in insect cell culture. This technique is also very useful in maximizing yields in a wide range of mammalian cultures. For system specifications or to request additional information, see www.nbsc.com/bih.htm.
You May Also Like





