Bio-Rad has launched a range of anti-ranibizumab antibodies for PK and immunogenicity assays on the back of increased Lucentis biosimilar development.
Bio-Rad Laboratories will offer customers a range of recombinant monoclonal anti-ranibizumab antibodies that are highly specific for Lucentis (ranibizumab), or the complex of ranibizumab with its target, vascular endothelial growth factor A (VEGF-A).
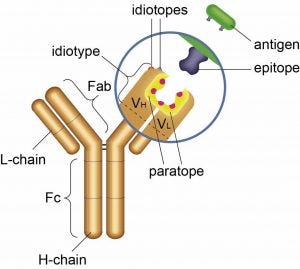
Antibody idiotope – the unique set of antigenic determinants of the variable region of an antibody
The anti-antibodies are to be used in pharmacokinetic (PK) and immunogenicity assays, and the product launch is due to the increased interest in Lucentis biosimilar development, Amanda Turner, product manager of Bio-Rad’s Life Science Group told BioProcess Insider.
“The demand for anti-ranibizumab antibodies comes from pharmaceutical companies developing a biosimilar of ranibizumab and from clinical research organisations providing services to support this biosimilar development,” she said.
“The antibodies are required for PK and immunogenicity assays as part of the totality of evidence required to demonstrate that there are no clinically meaningful differences between the biosimilar product and the original reference product in terms of the safety, purity, and potency.”
Developed and marketed in the US by Roche subsidiary Genentech, Lucentis is a monoclonal antibody fragment (Fab) approved to treat the ‘wet’ type of age-related macular degeneration (AMD), a form of age-related vision loss.
Last year, Lucentis sales pulled in CHF 1.4 billion (US$1.4 billion) for Roche, and $1.9 billion for fellow Swiss biopharma firm Novartis, which holds the marketing rights outside of the US.
With Lucentis patents expiring in 2020 in the US and 2022 in Europe, biosimilar developers – including Formycon, Pfenex and Xbrane – are lining up to take a slice of the market, said Turner.
“The demand for anti-ranibizumab antibodies has increased as the patent expiry draws closer.”
Anti-ranibizumab antibodies
While there are some pre-made assay kits available on the market, Turner said Bio-Rad’s anti-ranibizumab antibodies are unique:
“We have made four new recombinant, monoclonal antibodies including a highly innovative drug-target complex specific reagent that only recognizes ranibizumab when in complex with its target VEGF-A,” she said.
As a Fab fragment – as opposed to a full-length antibody – ranibizumab PK assay design is challenging because the format of the drug is incompatible with the PK bridging assay, commonly used with bivalent, full-length monoclonal antibody drugs, she continued. Additionally, it is present at very low levels in patient samples and therefore a highly sensitive assay is required.
“Bio-Rad’s high affinity ranibizumab-VEGF complex specific antibody overcomes these challenges by enabling the setup of a highly specific and sensitive PK assay in the antigen capture format, instead of the bridging format, for the quantitation of free ranibizumab drug or its biosimilars in patient samples.”

schedl_b_and_w.jpg?width=100&auto=webp&quality=80&disable=upscale)


schedl_b_and_w.jpg?width=400&auto=webp&quality=80&disable=upscale)





