Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
May 17, 2016

WWW.GRAPHICSTOCK.COM REPRINT WITH PERMISSION
A strategic technology transfer plan is the touchstone of global biomanufacturing enterprise, especially for contract service providers that must meet the needs of customers located across several continents. Like their clients, contract development and manufacturing organizations (CDMOs) are facing shortened timelines and cost pressures. They are turning to their process engineers and technology transfer teams to ensure communication with sponsor companies and streamline the transfer of information and critical activities between process development (PD) and manufacturing.
In his presentation at the 2016 BioProcess International West conference, Paul C. Jorjorian, director of global technology transfer at Patheon, highlighted the company’s approach for technology transfer across its global network of manufacturing and development sites. Here, he shares the key points of that presentation, including the company’s best practices and lessons learned.
Background
What are some of the company’s key parts of its global network? Traditionally, Patheon has been a world leader in drug product and small-molecule active pharmaceutical ingredient (API) manufacturing. Recently, the company acquired a number of companies focused on biologics bulk drug substance manufacturing, transforming the company into a CDMO that offers end-to-end services for clients seeking biologics and small-molecule manufacturing. As part of the expansion, Patheon acquired three biologics CDMOs: DSM Biologics, Gallus BioPharmaceuticals, and Laureate Biopharma (previously acquired by Gallus). Substantial efforts have been made over the past year to incorporate the best practices of those three companies under the umbrella of a fourth company (Patheon) to have a cohesive technology transfer platform. In total, the global network consists of two development sites and three manufacturing sites. This distribution of activities necessitates a technology transfer platform that is robust and efficient. My BPI West presentation focused on the systems Patheon has put in place to allow for rapid and robust process transfer between sending units (one of the two development sites) and the receiving units (the three manufacturing sites).
Ensuring a Balance
What is Patheon’s approach to technology transfer? A section from ICH Q10 is ubiquitously quoted when discussing technology transfer activities (see box, below) (1). Within Patheon Biologics, we view technology transfer as a two-way conduit between process development (PD) and operations. That technology transfer requires the flow of information from PD to operations needs little explanation. As a CDMO, we are not afforded the luxury to make substantial changes to our manufacturing infrastructure to facilitate each new process. As such, there needs to be information flow from operations back into the development laboratories. This condition results in a heightened awareness around design for manufacturability within the development laboratories. That said, technology transfer is not a one-way street, and the manufacturing teams do adapt to new technologies and prevent a “we’ve always done it this way” mentality. Tech transfer is a balancing act between PD and operations, and should foster communication and mutual agreement between the sending unit and the receiving unit.
At Patheon, this is achieved by using a common documented methodology by which processes can be transferred from a client to PD and subsequently, to manufacturing through a series of well-understood processes and subprocesses.
Technology Transfer Defined |
According to the ICH Q10 document, “The goal of technology transfer activities is to transfer product and process knowledge between development and manufacturing, and within or between manufacturing sites to achieve product realization. This knowledge forms the basis for the manufacturing process, control strategy, process validation approach, and ongoing continual improvement.” |
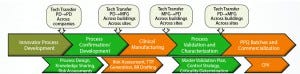
Figure 1: Technology transfer (process development to CGMP manufacturing) across all sites is the same as technology transfer within sites. BR = batch records, TTP = technology transfer protocol, CPV = continued process verification, PPQ = process performance qualification)
Would you provide an example of how those concepts can be implemented? The callouts across the top of Figure 1 show where technology transfers, associated with the process, typically occur throughout a molecule’s life cycle. You will notice as a CDMO, we are somewhat unique in the fact that there is an additional technology transfer that occurs. This is shown as a PD to PD transfer from the innovator company to the CDMO. It is critical process information and know-how be clearly communicated from the innovator company to the CDMO in a structured manner. If information is omitted or gaps in the original development are not identified at this early stage, it can result in unforeseen challenges during process scale-up or bench scale confirmation. Systems should be in place to ensure that all information that is needed from the innovator is being transferred effectively to the CDMO. We use a series of formalized checklists, templates, and risk assessments to ensure that this process happens in a proficient and consistent manner.
The next transfer point in Figure 1 is from PD to clinical operations, this is oftentimes occurring between different sites. Before this transfer and scale-up, it is typical that work within the development laboratories of a CDMO is complete. This can vary from cell line generation and development of a new process to simply confirming a client’s process using appropriate scale-down models. At this point, the development laboratory transitions from the receiving unit to the sending unit as the process moves from PD to manufacturing for clinical production.
Subsequent to that, there can be a transfer of knowledge back to PD after clinical manufacturing. This is the third transfer in Figure 1. This is usually in preparation for process characterization studies at the bench scale before process performance qualification (PPQ) batches and other process validation activities, the final tech transfer shown in Figure 1.
Design for Manufacturability
Your presentation highlighted two essential parts of a global approach for technology transfer. Would you review those? The first is facilitating the concept of design for manufacturability. During our integration journey, we found discontinuities in what each site viewed as the responsibilities of PD versus operations. Despite each previous company having good — in some cases fantastic — internal tech transfer capabilities, the challenges arose from each site having its own systems and knowledge base.
The first part of the approach focused on building a common understanding of each manufacturing site’s capabilities, including everything from equipment to raw materials and consumables to expectations regarding buffer and media preparation. It should never be assumed that process developers have an intimate knowledge of the equipment that’s being used in manufacturing if it’s not made available. This is especially true if the manufacturing or PD site is newly integrated. Because CDMOs can’t redesign a facility for every new molecule, this knowledge is critical. A development organization should have intimate knowledge of the receiving site’s capabilities. Without that, the development organization (through no fault of its own) will never be able to develop a process that will seamlessly and easily scale up.
The team also looked at how raw materials and consumables were being selected at the sending sites. Raw material qualification is a lot of work. It takes a great deal of effort to ensure that all vendors are qualified and audited, and that associated paperwork and documents are in place. Anything that can be done to limit the number of new raw material introductions will, in essence, streamline the process of tech transfer and new product introduction. It is also worth noting opportunities existed to reduce the number of buffer transfers. Each new buffer requires its own individual batch records. Beyond the required manufacturing documentation, the development group is required to come up with new formulations, typically acid–base conjugate pairs, as well as pH and conductivity specifications. All this adds up to more work for the organization, and lost time.
The second major focus area was around documentation. Each of the sites had their own documentation expectations, and associated standard operating procedures (SOPs) and policies for technology transfer. On the surface, the transfer documents were not very different. Despite this apparent early alignment we found, as with most things, the devil is in the details and clarity was needed on precise roles and responsibilities. Examples of those details included items such as document approval expectations, in-process filter sizing responsibilities, and ownership for downstream process sizing.
What can be done to make process design work more easily within a manufacturing network? Beyond multiple around-the-world flights for our scientists (one of our sites is in Australia), this was done in three ways. First, information was made easily accessible. Second and most important, communication was encouraged and facilitated. And third, clear expectations regarding responsibilities and documentation were set through a series of policy documents.
Information was made globally accessible through an online database. This database not only provides basic information such as equipment name and manufacturer, but also capability information. Whether that be the flow rate for a chromatography skid, the hold-up volumes for a ultrafiltration/diafiltration (UF/DF) system, the gassing strategies that are achievable by the various bioreactors at each one of the sites, and all of the other details that would be pertinent to a development scientist who must come up with a process.
As with the equipment, qualified raw material information was made available. Specifically, these raw materials lists were not just a list of everything that was qualified at each one of the sites; this type of list would be quite laborious to review. Raw material information is broken out by type, filters, bags, chemical, and so on, with the preferred or “platform” raw materials clearly highlighted. We put in quite a lot of work to align those raw materials as best we could globally, and this is still a continual effort within the network. It’s worth noting that preferred raw materials are made easily available in the PD laboratories, typically through kanban systems, as the processes are being developed. Although the introduction of new raw materials is unavoidable, limiting it to only the critical few provided smoother tech transfers and increased supply chain robustness.
For our case, we implemented this database through the company’s SharePoint site with all the information about preferred raw materials, buffers, and equipment. Any organization can do something similar, and I’m sure many have. There are a number of different ways it could be done. For example all that information can be hosted on a common drive.
Now as much as I’d like to say building databases and making information available will solve all your technology transfer woes, that is most certainly not the case. As with most things, a successful tech transfer boils down to communication. Therefore, we also made this an expectation within the project teams. Having PD engaging the process engineering and technology transfer teams early in the development process can prevent many headaches down the road. Through program management, the teams at the receiving site are engaged in conversations with PD as early as possible. This includes activities such as having the receiving site review the development plans. By doing that, we can identify new materials introduction earlier and get the associated paperwork started as well as identify potential facility fit constraints. Limiting in-process pool volumes during downstream processing is a common example of a fit constraint discussed during those early meetings. This strategy has resulted in fewer raw material introductions, fewer new buffer recipes, better facility fit, and more consistent processes within the development group.
How does Patheon fit that strategy into its design-for-manufacturability concept? I’d like to talk about how this concept applies in two different scenarios. In the first, we have programs that require full PD, where we are developing the process from scratch. In this case, it’s fairly easy to align raw materials, equipment need, and so on because you have a blank canvas for the PD team. So we can fully define all raw materials and processes, making the technology transfer process much more streamlined.
But much of the time, we are getting a second type of program: processes that have already been partially developed by an innovator company. In this case, we try to ensure that we’re aligning as much as we can on our platform. For example, we may align raw material vendors, bioburden reducing filter, operating methodologies for preculture steps, and chromatography column sanitization protocols. Those alignments allow us to better leverage our experience gained from a multitude of previous batches and better leverage our vendor relationships. That ultimately speeds the tech transfer process, and more important, derisks a customer’s programs.
Such decisions are not made in a vacuum. Process modifications such as vendor changes are reviewed with customers and are typically evaluated in development laboratory as part of a tech transfer process. Transparency and flexibility on the side of a customer and CDMO is critical for all programs, but especially in the case of a direct tech transfer. The ability of both sides to be open and honest during such early conversations is central in building the foundation to a successful program.
Customer involvement with both types of programs doesn’t stop at the development and tech transfer stage. Strong customer involvement all the way through a project’s life cycle is important. This can included everything from a person in plant (PIP) to establishing steering committees between senior management to help the project team remove any “roadblocks.”
Those are the two “bookend” scenarios we look at, but a lot of programs fall somewhere between full development and straight technology transfer.
Another topic I’d like to discuss is documentation. Let’s be honest, very few development scientists are eager to write a development report or a technology transfer document. Scientists like to be in a laboratory doing development work. I know that was always my preference. To help streamline the tech transfer document drafting, we’ve put in place a policydriven system using prebuilt templates. Internally, we call these “templated” transfer documents technology transfer protocols (TTPs).
Those documents facilitate the transfer from development to operations. We’ve made clear policy-driven expectations for what those documents should look like and have made templates available. Those blank templates are also housed in the same global site described above. They are like a series of building blocks, one per unit operation, that can be put together to form a cohesive document describing the process. Each unit operation has its own template that can be opened, populated, and copied and pasted into a fresh document. That eliminates the need to build a document from scratch. There is a difference between using a blank template and taking a completed document and changing it. I’ve always found that if you have a document for Project A and try to update for Project B, you oftentimes end up not deleting everything from the previous process. It’s very important that when you use templates, they are always blank when you start. In short, make information available and easy to find, encourage communication, and make documentation easy.
Accelerating Technology Transfer
What is another essential component of developing a global technology transfer strategy? Another key point is accelerating technology transfer. As a CDMO, no client has ever come to us and said, “Take as long as you’d like to make our product.” Clients — and more importantly patients — need those products as quickly as possible. With this in mind, we have looked at ways to streamline technology transfer processes by formally transferring key information in real time from PD to manufacturing, as opposed to transferring a fully defined process all at once.
Basically, instead of running PD activities (such as PD and confirmation) and manufacturing activities (such as raw material ordering and batch record drafting) in series, we make every effort to run them in parallel. However, when activities are run in parallel, you can end up with much more frequent crosstalk and interim deliverables that are required to make that process work.
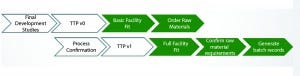
Figure 2: Process transfer; implementation of a staged approach to technology transfer of upstream and downstream processes, raw materials requirements, sampling plans, and buffer/ media formulations will save time but require diligence. White arrows are responsibility of development, and green arrows are responsibility of manufacturing. (TTP = tech transfer protocol)
Figure 2 is a high-level representation of how we transfer processes. This flow could occur unit operation by unit operation or for an entire process, depending on the timeline demands of a program.
Defining clear time-bound deliverables between PD and manufacturing is critical to that acceleration. We have found an Excel-based pre-TTP (which we call our TTP-V0) that can be used to push information to operations earlier facilitating long lead time activities. This document contains basic process information such as chromatography column sizes, media requirements, and preculture process flows. That information is often transferred before a process is fully “locked.” This early data are provided to allow staff within manufacturing to start planning and provides them more time to order and receive long lead-time raw materials. By the time development work is completed through a series of confirmation runs, we already have a good feel for how processes will be realized on the manufacturing floor.
Once PD confirmation runs are done, we write the full TTP, which is the formal document that’s signed off by the customers, operations, development, and so on. This formal transfer is typical for most companies. It is worth noting that dividing formal transfer into small subsections (even so far as unit operation by unit operation) can further accelerate tech transfer. For example, operations could be writing Protein A batch records, while viral filtration TTP is being drafted.
Do you have any final best-practice advice for CMOs? One of the key items that can’t be overlooked is the importance of everyone being on the same page regarding key handoff points within a technology transfer. It’s ultimately the people that make the difference; communication and collaboration is the cornerstone of any good tech transfer. Program managers play a key role in opening those lines of communication, but there must be accountability, trust, and buy-in from all sides — PD, QC, operations, and customers. Ensure that you’re hitting those key handoffs, whether they be TTPs, batch records, or raw materials ordering. Technology transfer is like an orchestra of different departments that all need to be providing key deliverables at the correct time, with the program manager acting as the conductor.
Acknowledgments
Paul Jorjorian thanks the development, technology transfer, and program management teams that contributed to the successful implementation of these systems, especially Evan Shave, David Jewell, Jaco Merwe-van-der, Liam Nolan, David Crowley, and Galyna Yakymechko.
References
1 ICH Q10: Pharmaceutical Quality System. International Conference of Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, June 2008.
Maribel Rios is managing editor of BioProcess International; [email protected].
You May Also Like