AbSolute® High Cap
August 1, 2012

The capture step in the downstream processing of monoclonal antibodies (MAbs) is often the bottleneck and the most expensive step due to the use of protein A media. In recent years, several new-generation protein A media have been launched on the market claiming improved MAb capture, or improved resistance to cleaning agents.
AbSolute® High Cap, the new revolutionary protein A media by Novasep, maintains excellent DBCs at low and high velocities, therefore, providing substantially improved productivity and strongly reduced costs compared with all existing protein A media.
Outstanding Dynamic Binding Capacity
AbSolute® High Cap combines the benefits of a new-generation Protein Awith rigid particles providing improved mechanical resistance. Due to a very uniform particle size distribution and a homogeneous pore size distribution, AbSolute® High Cap has outstanding performance at low and high velocities. As an example, DBCs at 10% breakthrough were determined for AbSolute® High Cap and several industry standards with human IgG in PBS at 0.5 mg/mL: AbSolute® High Cap provides much better results than market standards at all linear velocities.
Cleaning and Sanitization
AbSolute® High Cap is based on specially modified silica and has multiple-point protein A attachment to the matrix. Its resistance to high pH levels makes it stable through alkaline cleaning and sanitization. Indeed, AbSolute® High Cap remains stable after 400 cycles of use with alkali washing using 100 mM NaOH + 0.5 M NaCl every 10 cycles.
Highest Productivity and Economics
Based on experimental data obtained at lab scale, an affinity chromatographic step was designed to process 10,000 L of feed at 1.4 g/L of MAb in 16 hours. AbSolute® High Cap is incompressible meaning that a 1-L column is packed with 1 L of media. This feature represents an economical benefit in comparison with soft gels such as agarose-based media, which have compression factors of 1.25 and thus require a higher quantity of media to pack a 1-L column.
The geometry of all columns has been optimized, thus minimizing the volume of protein A required to perform the step, in order to maximize productivity. In these conditions, performing the step at industrial scale using AbSolute® High Cap allows a volume reduction in the media and an increase in productivity by up to 2.7-fold compared with other industry standards, resulting in a cost savings of up to $1,000,000 for the first load of media!
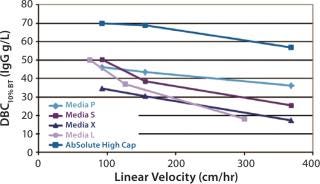
Figure 1: ()
Table 1: Productivity results for industrial applications
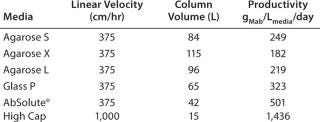
Table 1: Productivity results for industrial applications ()
About the Author
Author Details
Hélène Chochois is product manager biopharma, 81 Boulevard de la Moselle, BP 50, 54340 Pompey, France; +33-3-83-71-47; [email protected]; www.novasep.com.
You May Also Like





