Increased Load of DNA-Based Oligonucleotide on TSKgel SuperQ-5PW (20) Resin
August 1, 2011

Oligonucleotides are entering clinical trials in increasing numbers. In many ways, their entry into the clinic mirrors the development of protein therapeutics in the late 1980s and early 1990s. On the other hand, the purification process for an oligonucleotide therapeutic is very different from that for a protein. Typically one high-resolution, high-load step is used rather than a train of capture, intermediate purification, and polishing columns.
TSKgel SuperQ-5PW (20) resin — a 20-µm particle size anion-exchange medium — is becoming the “go-to” product for oligonucleotide purification. This article demonstrates the performance of the resin at higher loads while maintaining the resolution and purity of a target molecule.
Oligonucleotides are short, linear sequences of deoxyribonucleic acid or ribonucleic acid that are generally manufactured by chemical synthesis. Due to the unique structure of these molecules and the way they are synthesized, oligonucleotides require special considerations during chromatographic purification. During the synthesis of the oligonucleotide, there are a small percentage of sequences where a particular nucleotide may either be deleted or have more than one segment attached (N − 1 and N + 1, respectively, are the common nomenclature). Taken collectively, these synthesis errors may produce measurable amounts of impurities. The similarity in the impurities to the target molecule requires a high-resolution technique to adequately isolate a target molecule.
This report shows the power of TSKgel SuperQ-5PW (20) anion-exchange media to purify synthetic DNA-based oligonucleotides.
Objective
The objective of the study is to determine the ability of TSKgel SuperQ-5PW (20) resin to adequately purify crude oligonucleotide at loads >1 mg/5.13-mL column.
Experimental Methods
The phosphodiester deoxyoligonucleotide (20-mer) used in this study had the following sequence:
5′ – GAA TTC ATC GGT TCA GAG AC-3′
This oligonucleotide was purchased unpurified (estimated at 64.9% purity by HPLC) in lyophilized form from Trilink Biotechnology (San Diego, CA). The extinction coefficient was 199.9 OD units/µmol, and the molecular weight of the free acid was 6140.9 Da. This sequence was chosen to minimize the amount of secondary structure effects during the purification experiments. TSKgel SuperQ-5PW (20) resin was packed in a 6.6 mm ID × 15 cm column. The column was performance tested with NaCl (1% CV injection of 2 mol/L NaCl with a mobile phase of 1 mol/L NaCl) and found to be acceptable for use in these experiments.
For all experiments performed, the crude oligonucleotide was diluted into the column equilibration buffer (Buffer A) before loading onto the column. For a 1-mg load, 52 µL of crude oligonucleotide was diluted to 10 mL with Buffer A and loaded into the sample loop. For a 2-mg or 5-mg load, 104 µL and 260 µL, respectively, were used. All experiments in the 6.6-mm ID columns were run at 250 cm/h.
The buffers chosen for this set of experiments were
Buffer A: 20 mmol/L Tris, 1 mmol/L EDTA pH 9.0
Buffer B: 20 mmol/L Tris, 1 mmol/L EDTA, 1 mol/L NaCl pH 9.0
Buffer A served as the column equilibration and sample dilution buffer, and Buffer B served as the gradient elution buffer.
A modified step gradient previously established for purifying oligonucleotide was used on the TSKgel SuperQ-5PW (20) column. The established gradient for the resin is as follows and used throughout the experiments conducted for this report:
Step to 35% B (5 CV)
Linear gradient 35–53% B (10 CV)
Step to 100%B (5 CV)
Fractions were collected along the N − 1 peak, main peak, and N + 1 peak region and were analyzed using a TSKgel DNA-NPR HPLC column (4.6 mm ID × 7.5 cm) to check for peak purity. Fractions of the main oligonucleotide peak that were >85% pure were pooled together. This pool was then analyzed for overall peak purity and recovery. Recovery of pure oligonucleotide is determined by multiplying the total (mg) amount of material (N − 1, oligonucleotide, N + 1) in the pooled fractions by the percentage of pure oligonucleotide in that pool as determined by TSKgel DNA-NPR HPLC.
Results
TSKgel SuperQ-5PW (20) resin visually resolved N − 1 peak and N + 1 peak from the main oligonucleotide peak at 1, 2, and 5-mg loads (Figure 1).
The 97.04% purity of the main peak indicates that at a 5-mg load, the resin is not yet being loaded to its operational capacity (Table 1).
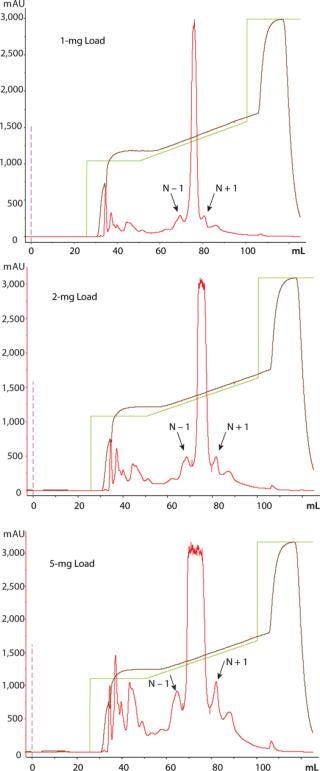
Figure 1: ()
Table 1: TSKgel SuperQ-5PW (20) resin at different loads of crude oligonucleotide
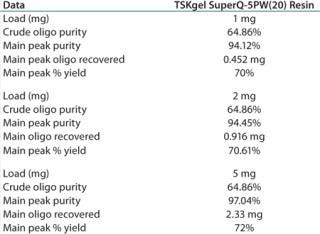
Table 1: TSKgel SuperQ-5PW (20) resin at different loads of crude oligonucleotide ()
Conclusions
TSKgel SuperQ-5PW (20) resin can separate oligonucleotides at loads >1 mg and potentially >5 mg/5.13 mL column.
TSKgel SuperQ-5PW (20) maintains excellent main peak purity even at higher loads.
You May Also Like





