Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
August 1, 2011

Biopharmaceutical companies are increasingly interested in single-use innovations to improve performance and alleviate up- and downstream bottleneck constrictions. High-priority considerations for improvement include decreasing the number of process steps, reducing the risk of cross-contamination, and achieving a higher sterility assurance level. One bottleneck today is a need to collect QC samples at various steps during biopharmaceutical commercial-scale cGMP manufacturing, which typically is a labour-intensive manual activity that holds a potential risk of contaminating product or personnel.
Alfa Wassermann Separation Technologies (www.awst.com) is a leading provider of industrial process equipment for biopharmaceutical manufacturing. it recently launched eMPat™, a fully automatic aseptic QC sampling system especially designed for biopharmaceutical cGMP manufacturing. The compact EMPAT system is designed for flexible and reliable operation and seamlessly fits with existing QC sampling planning.
Table 1: Specifications of the EMPAT™ aseptic QC sampling system

Aseptic QC Sampling
Flow-Path and Sterile Connection: The EMPAT system sampling flow-path is a sterilized disposable multisample bag set for seven samples, which is easily mounted on the EMPAT system and will stay connected during an entire day (batch). The bag set assembly inlet tube ends with a sterile Kleenpak™ connector, which is aseptically connected to a production vessel or line. After connection is established and a sampling plan for that batch is selected from the menu, the EMPAT system automatically collects multiple QC samples of user-defined volumes and at user-defined intervals during the entire batch run, without the need for additional intrusions to the production vessel or line.
Reproducible and Reliable Results:
Reduced risk of contamination or sterility breach, assured product sterility at all times
Reduced risk of subjecting operators to hazardous materials
Elimination of QC sampling errors, minimizing risk of delay in product release by having to investigate false positive QC results
No cross-contamination between samples, improving the quality and reproducibility of QC sampling
Reduced need for paper-based sampling schedules, records, and notes
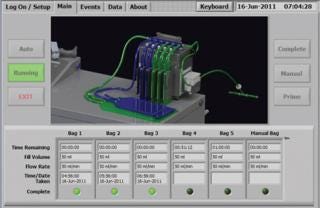
Quality by Design Features:
E-signature, user name log-in and multilevel password authorization
Menu selection of preprogrammed sampling protocol
All operating and batch data is stored in an SQL database
Batch data automatically archived (critical process parameters, events and alarms)
Report generator included, data communication enabled (Ethernet and USB)
IP65 touch screen, bar code reader, and bar code printer included
The system is operated by plug-and-play Windows platform software and is designed for paperless operation with 21 CFR and quality by design in mind.
About the Author
Author Details
Rienk de Bruijn is vice president of sales and marketing at Alfa Wassermann Separation Technologies; 31-348-487-300; fax 31-348-433-000; [email protected].
You May Also Like