Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
To enable broad, global access to life-saving biopharmaceutical products, our industry is facing significant pressure to reduce the overall cost of manufacturing and enable local manufacturing where possible. Combined with growing markets outside the United States and Europe and development of high-titer, high-yield processes, that pressure has led to a shift in the industry’s approach to facility design and construction. Today’s biopharmaceutical production facilities must be flexible, cost effective, and readily constructed with minimal capital investment and construction timelines. As available single-use products for biopharmaceutical manufacturing have advanced and modular facility design and construction have improved, the industry has developed modular, flexible biomanufacturing facilities that can be easily replicated in multiple locations.
We discussed advantages of combining single-use technologies with modular construction in the first of this three-part series (1). Here we present an economic model for a typical monoclonal antibody (MAb) platform production process along with a detailed comparison of the operating costs for that process in facilities designed and built using either conventional reusable stainless steel equipment or single-use technologies to the maximum extent possible. We also discuss general concepts and design features for a standard facility designed to produce commercial MAb quantities using the platform process.
Design Basis Overview
Our conceptual facility design and economic analysis is based on the MAb platform process outlined in Figure 1. We based our cost analysis on a cost-of-goods (CoG) model performed using the industry-standard BioSolve cost-modeling software, version 4.0, available from Biopharm Services Ltd. (2).
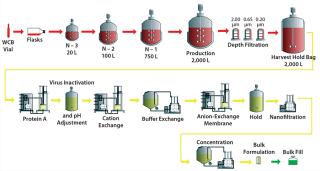
The upstream portion of this platform process begins with cell expansion from a vial of the working cell bank (WCB), cultured through a series of T flasks, shaker flasks, and small-scale bioreactors up to 500 L, followed by product production and accumulation in a 2,000-L bioreactor. The facility design allows for installation of bioreactors from anymanufacturer(s). After cell growth and protein production in the 2,000-L bioreactor, its contents are clarified through a combination of depth filtration and membrane filtration unit operations to yield a clarified cell culture harvest containing crude MAb product.
That product is then purified from the clarified harvest using a three-column purification process including protein A affinity chromatography, cation-exchange (CEX) chromatography, and anion-exchange (AEX) chromatography. A low-pH virus inactivation step follows the protein A column. A nanofiltration step follows chromatography for additional viral clearance, after which purified MAb product is concentrated and formulated by a combination of ultrafiltration and diafiltration (UF/DF) to produce the final purified and formulated bulk product.
That purified, formulated bulk MAb will be filled into standard 10-mL glass vials in a separate drug-product manufacturing process. Assuming a high-expression cell line producing 5 g MAb per liter of bioreactor harvest with industry-standard purification yields (3), this standard platform process should produce about 7 kg of purified bulk MAb product per batch. That will be filled into about 60,000 vials.
Facility Design Parameters
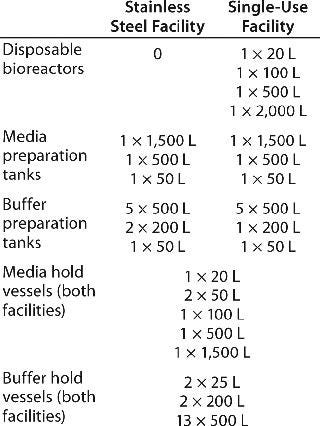
Using the platform process described above, we modeled two different facilities for MAb production. Although we refer to it as the stainless steel facility, the first consists of a hybrid design in which all seed and production bioreactors as well as mixing vessels for buffer and media preparation are reusable stainless steel vessels. But disposable bags (≤2,000 L) are used for storage of media, buffers, and product intermediates. Each disposable plastic bag used in those applications is held in a portable, stainless steel system designed to hold bags of a desired volume. Systems used for product and intermediate storage were also equipped with disposable mixing systems (where appropriate) to keep the product well mixed. Single-use mixing systems typically involve a magnetic or mechanical external drive with a disposable impeller inside the bag.
The second facility, referred to as the single-use facility, is equipped with disposable seed and production bioreactors. In addition, all media and buffers are prepared in single-use bag systems consisting of powder transfer bags, disposable bags with a disposable internal agitators, external mixing systems, a weighing station, and a disposable path (pump, tubing, filters, and so on) for transfer of prepared media or buffers into another disposable bag system for storage. When necessary, buffers would be prepared in concentrated solutions to accommodate transport in disposable bags at a maximum volume of 500 L, which is a typical volume limitation for longer-distance transport within a facility. In-line dilution skids are placed at point of use for concentrated buffers.
Media in both facilities are made at the start of each production batch. Similarly, buffers in each facility are prepared in advance and stored within the facility until needed. A new batch of media or buffer would be prepared for each production batch. Table 1 details the number and type of vessels used for cell culture production, media and buffer preparation, and storage in each model facility.
Table 1: Number and size of tanks required for media and buffer preparation and storage
We used traditional equipment in both models for all recovery and downstream process (DSP) unit operations. Each chromatography step included in the purification process uses 63-cm diameter columns packed to 20-cm bed heights, with each column being used for multiple cycles per batch. For the protein A affinity column, five cycles are required per batch. The CEX column uses three cycles per batch, and the AEX column uses two. We set the titer for both production reactors at 5 g/L, with an overall yield of purified bulk MAb at 70%.
The total time required for the cell culture portion of this MAb process is about 32 days — from thawing of a WCB vial to the production culture’s end, with a 15-day residence time in the production bioreactor (Table 2). In the stainless steel case, an additional three to four days are required at the end of
the production process for bioreactor cleaning and resterilization, making the production bioreactor a rate-limiting step for the overall process. Although the turn-around time for a single-use bioreactor is not instantaneous, it is much shorter (typically no more than one day) because it involves simply removing a used bioreactor and replacing it with a new one.
Table 2: Upstream timing for MAb production

Process Economic Analysis
Taking into account the bioreactor process time and that required to clean and resterilize a stainless steel bioreactor, total turn-around time for a stainless steel production bioreactor can be two weeks or more. So our facility model is based on the maximum number of stainless steel bioreactor runs possible in a year. Assuming an annual two-week facility shut-down, about 90% use of production bioreactor capacity, and one failed batch per year, a facility based on stainless steel bioreactors could complete 15 batches each year.
To properly compare the cost and output of our model single-use facility with those of the stainless steel model, we fixed the number of batches in the former at 15 MAb batches per year. In reality, the shorter turn-around time for single-use bioreactors could allow production of about 19 batches per year, which increases the productivity of a single-use facility and lowers its overall operating costs per batch.
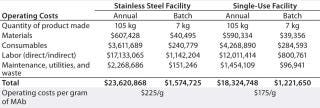
Based on the standard platform process described above with 15 successful product batches each year, Table 3 compares estimated operating costs for the two model facilities. “Materials” include the cost of cell culture media, buffer components,process water (PW), and water for injection (WFI). Prices are based on catalog prices for standard, commercially available versions with anticipated volume discounts.
Table 3: Operating costs (in US dollars) for stainless steel and single-use facilities
“Consumables” in our model include such items as
filters preparing buffers and cell culture media as well as filtering intermediates during production
disposable bags preparing and storing cell culture media, buffers, product intermediates, and final product
disposable bioreactors
ultrafiltration membranes used for UF/DF
chromatography media used in the two column-chromatography steps
membrane adsorbers used for the final polishing step in the process.
For those consumables, we used the average price for each item based on standard pricing from different vendors, again allowing for volume discounts. And we used the amortized cost of chromatography media, taking into account the number of cycles for which each column would be used before being repacked.
“Labor” costs are based on average US labor rates for operators, supervisors, and quality assurance and control (QA/QC) staff in the New England area. “Maintenance” costs include stocking spare parts and labor associated with equipment upkeep as well as the overall cost of maintaining a facility in a clean and GMP-ready condition. “Utility” costs include electricity to operate a facility as well as the cost of natural gas required for heating and steam generation. All costs associated with handling and disposal of contaminated and uncontaminated aqueous and solid waste are included under “Waste,” along with the cost associated with disposing of plastic bags and other single-use components.
With both facilities producing the same number of batches using the same production process, each is capable of making 7 kg MAb per batch — for a total of 105 kg of bulk MAb per year. The cost advantage of using disposable bioreactors, however, is evident in about 22% savings in total operating cost per batch for a single-use facility compared with a stainless steel facility. Comparing detailed costs for different components of the overall manufacturing costs for these two model facilities, those associated with materials, labor, and maintenance/utilities/waste for the single-use facility are lower than those for the stainless steel facility, but the consumables cost is higher.
Material cost savings for the single-use facility are driven primarily by savings associated with eliminating bioreactor cleaning and sterilization. A single-use facility also eliminates PW, WFI, and chemicals such as phosphoric acid and sodium hydroxide for cleaning stainless steel bioreactors, which generates a significant cost savings. That comes primarily from the different size and types of PW and WFI systems needed for each facility. In the single-use facility, a relatively small but rapid water generation source is required rather than the slower but much larger system for the stainless steel facility, and the water system must produce less water overall. But if the single-use facility were to run at its maximum capacity, it would require a faster water-generation rate than would the stainless steel facility because of the shorter duration of each batch. So the PW and WFI systems in the single-use facility actually would be more expensive per liter of water produced.
The consumables cost difference for these two model facilities is entirely attributable to the cost of disposable bags. Single-use systems for media and buffer preparation and storage — in addition to the disposable bioreactor bag systems used in cell culture for each batch of monoclonal antibody — yield about $40,000 higher consumable costs per batch for the single-use facility. That includes the cost of bags used in an assumed one failed batch per year, then spread out over the 15 successful runs. If the single-use facility runs at maximum capacity (limited to one failed batch per year), then the cost of disposable bag systems per gram of product produced would be lower.
The lower maintenance/utilities/waste costs for the single-use facility are primarily attributable to elimination of maintenance costs associated with stainless steel bioreactors. Also, about 20% of the cost savings in this category come from reduced use of clean steam and power for cleaning and sterilizing those bioreactors. Of note is the almost negligible cost difference for waste disposal between these two model facilities. That’s because the cost of disposing larger amounts of solid waste associated with a single-use facility is offset by the disp
osal cost of the larger quantities of liquid waste generated by the stainless steel facility.
Those results are consistent with a comprehensive study of the environmental impact of single-use systems, which showed significant reductions in energy demand and water use for media preparation and buffer preparation as well as cell culture in a single-use facility compared with a stainless steel facility (4, 5). Rawlings and Pora concluded that a single-use facility is about 50% less energy intensive due to significantly lower consumption of energy otherwise required to heat large volumes of water for cleaning and sterilizing stainless steel equipment (6). In their study, the cost of plastic bag disposal in a single-use facility was offset by the energy recovery gained through their incineration.
As noted above, we restricted productivity of the single-use facility in our model to the same number of batches per year as the stainless steel facility. But in reality, a single-use facility with the same size bioreactors as a stainless steel facility can produce a higher number of batches each year. Time saved by eliminating bioreactor cleaning and sterilization — along with shorter turn-around times — allows for completion of 20 batches/year in a model single-use facility, for a total production rate of 140 kg MAb /year. This large number of batches effectively lowers the operating CoG for the single-use facility to $170/g when it operates at full capacity — a much larger cost savings for the use of disposable bioreactors and other such systems.
One key potential benefit derived from implementing single-use technologies in biomanufacturingoperations is a reduction in labor requirements for supporting ongoing GMP manufacturing. Such reductions come primarily from lowered requirements for equipment cleaning between production batches and associated ongoing cleaning validation/verification. Those reduced cleaning requirements can not only lower headcount for direct manufacturing operations, but they may significantly reduce staffing requirements for QA/QC as well. Also, shorter change-over times and fewer hard-piped utilities and systems in a facility incorporating single use technologies will lower maintenance and metrology costs, reducing staffing requirements for those areas as well.
Our model shows significant cost savings in labor required to operate a single-use facility compared with a stainless steel facility — primarily due to time saved in each production batch and the shorter batch turn-around times. Table 4 lists staffing estimates based on labor requirements for a fully operational facility using output from our process economic model and recent benchmarking studies performed by BioProcess Technology Consultants. As shown, a single-use facility requires about 15% fewer manufacturing staff for drug substance manufacturing and about 12% fewer QA/QC staff, resulting in about 13% lower total headcount than a stainless steel facility. Those staffing reductions are consistent with other reports of reduced labor requirements for facilities based on single-use technologies (7,8,9). For example, in a case study by Foulon et al., estimated labor savings for technology transfer and ongoing production of a MAb product in a single-use facility was 56% for technology transfer and 17% for ongoing production compared with conventional stainless steel facilities (10).
Table 4: Full-time employee (FTE) staffing estimates for a typical MAb facility

Our modeling results show additional labor savings in the single-use facility because it is running at only 75% capacity to match the output of the stainless steel facility. That lower capacity use consequently lowers labor requirements. If the single-use facility were to run at full capacity, then its total headcount would be slightly higher, but so would the total amount of product made per year.
The primary difference between the single-use and stainless steel (hybrid) facilities manifests in the use of disposable bioreactors rather than stainless steel. Downstream processes and equipment are essentially the same in both facilities. However, as more single-use technologies are developed for downstream processing and such technologies become routine for good manufacturing practice (GMP) manufacturing, we expect the overall cost of manufacturing to decrease even further. In a recent presentation, for example, Malcolm recently showed that about 75 labor hours/batch could be saved using disposable prepacked chromatography columns instead of reusable, self-packed columns (11). Those prepacked columns reduced the total cost of each chromatography step some $20,000.
Next-Generation Modular Facilities
Based on the platform process described herein, we have developed a standardized facility layout for a modern MAb production facility that can be readily adapted to produce other recombinant protein products and different MAb processes. The layout and cost of this standardized facility design will be detailed in a future article. The design consists of several different functional areas interconnected by a central spine or corridor to allow for maximum flexibility and ease of future expansion.
Functional areas incorporated in the standardized facility include administration, QC, QA, manufacturing, and materials handling and storage (warehouse). An optional process development laboratory could also be included. Space for the administration, QC, QA, and materials-handling functions can be built using conventional construction. The manufacturing area is modular in construction both for bulk drug substance and final-dosage manufacturing, and it can be installed either in an existing or new building shell or as a free-standing structure using modules specifically constructed for this purpose.
Bulk Drug-Substance Manufacturing: Our standard biomanufacturing facility for bulk MAb production includes appropriate space for material staging and dispensing, media and buffer preparation and storage, and upstream and downstream processing, along with sufficient space for all support functions and appropriate airlocks and corridors. Although the standard design is based on a single 2,000-L disposable production bioreactor, the layout can be adapted for bioreactors of different sizes or readily expanded to include multiple 2,000-L bioreactors.
By maximizing the use of disposables in upstream processing and product storage, the overall footprint of a bulk MAb production facility is about 1,200 m2. The standardized facility incorporates modern requirements and approaches to design and construction, including modular construction. If manufacturing is based on reusablestainless steel equipment instead, then the size of the facility increases ~50% to 1,800 m2. The smaller footprint of a single-use facility primarily comes from the smaller utilities needed to operate such a plant, which further helps to reduce overall facility cost.
The facility design for bulk MAb production includes unidirectio
nal flow of materials, product, waste, and personnel throughout the manufacturing area. In addition, separate processing areas are provided for downstream processing operations before and after virus removal by nanofiltration. Fully closed and contained processing is used wherever possible, generally within a grade D environmental classification. Open processing areas —as required for inoculum preparation, final purification, and bulk filling — are designed to be grade C, with specific open operations performed in suitable biosafety cabinets using laminar air flow. The facility also includes suitable staging areas for raw materials, consumables, and equipment as well as appropriate locker rooms and airlocks for staff changing and entry/exit from the facility. To further ensure segregation within the facility, our design includes multiple air handlers. Using modular construction, the overall facility design can be readily modified to achieve appropriate segregation based on an appropriate risk assessment of products to be made, details of their manufacturing processes, and risks of cross-contamination or contamination from adventitious agents.
The product processing area is generally U-shaped, with unidirectional product flow from one end of the facility to the other. Media and buffer preparation areas are located in the center of the facility to allow for the most possible adjacencies to processing areas. Wherever possible, buffers are stored in closed containers within controlled but unclassified space to minimize the environmental burden and lower overall heating, ventilation, and air-conditioning (HVAC) requirements for the facility. The facility also includes optimized equipment positioning to minimize tubing and piping needed for product and material transfer. A single access point is provided for all production personnel, with a surrounding clean corridor for easy access to all rooms.
Final Drug-Product Manufacturing: A final-drug product manufacturing facility would be built using modular construction, with
a core area for product formulation and vial filling, stoppering, capping, and loading onto trays
space for vial and stopper washing, sterilization, and depyrogenization
necessary areas for material dispensing, washing, and sterilization of parts.
This module is equipped with appropriate internal air locks and corridors and independent HVAC units, all on a single level. Including space for personnel gowning and degowning, lockers, and so on — as well as ingress and egress airlocks — the drug product module will have a total footprint of ~330 m2. If lyophilization is required, it can be in the same module, adding ~60 m2 to the total facility size.
For drug-product manufacturing, a vial-filling line has the capacity to fill 6,000 10-mL vials/hour (~40,000 vials/day) for a single shift operation. Our model MAb process will make sufficient product to fill ~64,000 vials, so filling an entire production batch would require two days of filling operations. Allowing time for preparation, inspection, labeling, and so on, it would take about a week to fill the total monoclonal antibody output from a single 2,000-L bioreactor.
Economics of Modular Facility Design and Construction
According to Gilroy and Martini, “modular construction” of a pharmaceutical manufacturing facility refers to construction of all or part of a new or renovated facility built at a remote location, transported to the owner’s address, and reassembled on site (12). Modules consist of structural frames that are fit out with all mechanical, electrical, and plumbing architectural elements — complete with all fixed process equipment.
Estimating the cost of design and construction for such a modern biopharmaceutical manufacturing plant is a complex task that requires careful consideration of all associated time and risk factors. Differences in time required for such critical activities as architectural and engineering design and construction management — or the increased interest charges on applied capital resulting from project delays — can significantly affect the overall cost of building a biomanufacturing facility. A full risk assessment of timelines and costs comparing traditional and modular construction approaches shows that the risk mitigation and exemplary performance associated with modular construction makes that option the more cost-effective approach (13). As Figure 2 shows, the time savings is on average over a year. Furthermore, taking into consideration the net present value (NPV) of the potential revenues from products made in a given manufacturing facility will further reduce overall capital cost of design and construction. That increases the value of the modular approach.
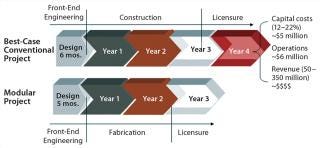
One key advantage to modular construction for biopharmaceutical facilities is the off-site construction of modules. The benefits of such an approach include enhanced quality control, reduced waste, reduced impact on current operations, and simplified site logistics. Transferring labor hours away from the construction site also reduces both risk and overall cost for a facility construction project. For example, building multiple modular elements in parallel with no weather impacts can reduce the construction schedule for a facility project by ≤50%. And the ability to leverage factory acceptance testing (FAT) at a module construction facility will often shorten the start-up and commissioning of a new facility.
By designing and building the process and facility modules needed for a biopharmaceutical facility, FAT and prequalification of all process equipment and utilities can be performed before shipping the modules. Once those modules are delivered to the construction site, they are assembled into the final facility so that final testing and qualification can be completed. Today’s modular wall systems evolved from prefabricated polyvinyl chloride (PVC) sheathed aluminum-frame wall and ceiling panels. They offer a high degree of flexibility, including“walkable” ceilings and prefabricated return-air walls. Modular wall systems can incorporate integrated electrical lighting and receptacles, HVAC ductwork, high-efficiency particulate air (HEPA) filters, and controls (12).
In a study on energy costs for both single-use and stainless steel facilities, Rawlings and Pora concluded that the main contribution to the life-cycle cost of a biomanufacturing facility comes from total energy costs incurred in running it (6). For a traditional biopharmaceutical manufacturing facility, ~70–80% of total energy consumption comes from numerous HVAC systems required to maintain a clean environment in the manufacturing areas. Using an optimized room layout and environmental classifications, the total energy consumption for a modular facility can be reduced by ≤15%. Furthermore, using modern manufacturing technologies and prefabricating facility modules off-site in a controlled environment provides for a higher-quality facility with tighter seals, lessening the chance of air leakage and energy loss. That in turn reduces the factors on differential pressure
s and air changes, leading to further energy reductions.
Using a system of nonproduction-hours setback (with the number of air-changes reduced during nonproduction hours that involve low particle burdens while maintaining correct air quality and pressure cascades among rooms) also reduces a facility’s overall energy requirements. As a result, the efficient design achieved using disposables and modular construction provides for a modern biomanufacturing facility that is consistent with sustainability programs such as Leadership in Energy and Environmental Design (LEED), Energy Star, and Building Research Environmental Establishment Assessment Method (BREEAM). This modern design is also consistent with ISO 14001, allowing for efficient environmental management and control systems.
Manufacturing plants will increasingly involve modular building strategies coupled with maximal use of disposable process equipment and lean design concepts. These approaches and technologies will provide significant cost savings and greatly reduce facility start-up times. They incorporate quality-by-design (QbD) concepts into facility designs, which should further reduce costs, increase efficiencies, and ensure regulatory compliance. When combined with single-use technologies for biomanufacturing, modular construction helps reduce facility operating costs overall. Savings resulting from the use of disposables installed in a low-cost modular building — as well as the significantly reduced construction schedule — will enable rapid construction of energy-efficient, economical, biomanufacturing facilities in both established and emerging markets.
About the Author
Author Details
Corresponding author Howard L. Levine is founder, president, and principal consultant of BioProcess Technology Consultants, Inc. (12 Gill Street, Suite 5450, Woburn, MA 01801-1728; 1-781-281-2703; [email protected]), and founder and CBO of Biocrescentia LLC (1-781-281-2703; [email protected]). Rick Stock is a consultant for BioProcess Technology Consultants, Inc. Jan Lilja is commercial director and Åsa Gaasvik is Senior Design Engineer at KeyPlants AB in Sweden. Hans Hummel is director, business development at KeyPlants in Switzerland. Thomas C. Ransohoff and Susan Dana Jones are vice presidents and senior consultants with BioProcess Technology Consultants, Inc.
1.) Levine, HL. 2012. Efficient, Flexible Facilities for the 21st Century. BioProcess Int. 10:S20-S30.
2.) Sinclair, A, and M Monge. 2009. Disposables Cost Contributions: A Sensitivity Analysis. BioPharm Int. 22:14-18.
3.) Lewis, DB Levine, HL and G 2010.BioProcess Technology Consultants, IncThe Development of Therapeutic Monoclonal Antibody Products, Elanders Sverige AB, Molnlycke.
4.) Mauter, M 2009. Environmental Life-Cycle Assessment of Disposable Bioreactors. BioProcess Int. 8:18-28.
5.) Pietrzykowski, M. 2011. An Environmental Life-Cycle Assessment Comparing Single-Use and Conventional Process Technology. BioPharm Int. 24:S30-S38.
6.) Rawlings, B, and H Pora. 2009. Environmental Impact of Single-Use and Reusable Bioprocess Systems. BioProcess Int. 7:18-26.
7.) Hodge, G 2004. Disposable Components Enable a New Approach to Biopharmaceutical Manufacturing. BioPharm Int. 17:38-49.
8.) Hodge, G. 2009.. The Economic and Strategic Value of Flexible Manufacturing Capacity.
9.) Fromison, J 2009. Disposables in Clinical Manufacturing. Am. Pharmaceut. Rev. 12:20-27.
10.) Foulon, A. 2008. Using Disposables in an Antibody Production Process: A Cost-Effectiveness Study of Technology Transfer Between Two Sites. BioProcess Int. 6:12-17.
11.) Malcolm, F. 2013.. Implementing Disposable Chromatography-Process and Technology Fit.
12.) Gilroy, J, and G Martini. 2012. Modular Construction Considerations. Pharmaceut. Proc. 27:22-23.
13.) Jameson, P. 2007.. Modularization: Is It Right for You?.
You May Also Like