Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
Development of industrial cell culture processes for production of recombinant proteins seeks high efficiency, reproducibility, and predictability. Usually the time allowed for process development is short, during which culture conditions and scale-up protocols must be defined so as to maximize cell productivity and yield while minimizing process scope and overall costs (1).
Although scientific literature describes various methods that increase productivity of a cell culture by reducing and arresting cell growth or weakening cell physiology (2), the cells must be in good physiological state at the beginning of each process so that long and costly preproduction scale-up steps will be unnecessary. Furthermore, those in a poor physiological state may respond unfavorably to stresses occurring in industrial-scale bioreactors, which emphasizes the central role of cell physiology in successful process development (1).
PRODUCT FOCUS: ALL BIOLOGICS
PROCESS FOCUS: PRODUCTION
WHO SHOULD READ: QA/QC, PROCESS DEVELOPMENT, AND MANUFACTURING
KEYWORDS: PRODUCTION CONTROL, PAT, ATP, OPTIMIZATION
LEVEL: ADVANCED
In recent years, considerable effort has been made to establish suitable online process analytical tools for animal cell culture process control. Online flow-injection analysis in combination with photometric, amperometric, and enzymatic methods as well as automated offline analyses such as mass spectrometry, gas analysis, and flow cytometry offer great potential for monitoring biochemical process variables (3,4,5,6,7). Although established analytical methods deliver useful data, they are incomplete because they only partially address the cellular metabolic state and also because no predictions can be made (1).
For instance, counting cells allows only a post facto assessment of their state because cell numbers simply reflect an existing physiological state. Cell viability reveals nothing about the state of nonviable cells. Determinations of metabolic byproducts correlate well with decreased cell growth/death but cannot provide full insight into the reaction kinetics of such changes — and provides even less about the real limits of the metabolites. Substrate (glucose) uptake and amino-acid consumption measurements give limited information on cell metabolism and can be regarded merely as rough indicators of cell physiology (1). An oxygen uptake rate (OUR) correlates well with viable cell concentration; however, it alone cannot predict the outcome of a culture (8).
An intracellular variable is needed to supplement those above-mentioned methods and enable more detailed monitoring of the physiological state of cells in culture. A promising option is found in universal-energy biochemical reactions, which are extremely important for cell metabolism (9,10,11,12). As early as 1968, Atkinson et al. postulated the energy charge (EC) hypothesis of adenine nucleotides and their central importance in correlating metabolic sequences and maintaining the metabolic stability necessary for life with Equation 1 (13).
In vivo measurement of cellular EC (mostly for microorganisms) demonstrated that growth can occur only at energy charge values around 0.9 and that cells die at values below 0.5 (14, 15).
We aimed to establish and evaluate an assay for rapid and reliable online measurement of intracellular ATP (adenoside-5′-triphosphate) to monitor the physiological state of cells in combination with other parameters (cell number, viability, and glucose consumption). Moreover, we wanted to adapt the system to an automated analyzer and thus transfer cell sampling, sample processing (pulping), diluting, and data collected. Biospectra AG (Schlieren, Switzerland) produced the analyzer, addressing the challenge of developing a liquid system that can cope with cooled liquids in small volumes. To assess the application of the resulting device, test runs were performed at the Zürich University of Applied Sciences (Züricher Hochschule für Angewandte Wissenschaften, ZHAW) in Winterthur, Switzerland. A second test phase demonstrated its successful implementation in bioreactor process monitoring at Novartis Pharma AG (Basel, Switzerland).
Bioreactor Set-Up
A number of in-house designed 15-L glass bioreactors was equipped with a magnetic coupled stirrer and probes for online determination of dissolved oxygen (DO), pH, and temperature. Live-cell concentration probes from Aber Instruments Ltd. (www.aber-instruments.co.uk) were also fitted to monitor viable biomass. Inlets for media or feed dosage and outlets for waste and sampling were also available. A thermal mass-flow controller connected to a ring sparger guaranteed continuous aeration and a constant gas flow-rate. A peristaltic pump (sterile barrier) withdrew samples for amino acid determination by online high-performance liquid chromatography (HPLC) every 60 minutes and for the ATP-analyzer every 176 minutes.
Cells and Culture Conditions: During studies at the ZHAW, Chinese hamster ovary cells (CHO, CRL-10762 from Cambrex Bio Science Walkersville, Inc., www.cambrex.com) were cultured in 15-L bioreactors with a protein-free medium (Gibco CD-CHO medium from Invitrogen GmbH, www.invitrogen.com) under defined conditions: pH 7, minimal gas flow 50 N/h, pO2 60%, O2 headspace gassing, temperature 37 °C, and stirrer speed 130 rpm.
At Novartis, SP2/0 cells (a hybridoma cell line) were expanded and grown in a RPMI-based medium. During inoculum preparation, cells were maintained in T-300 cell culture flasks at 100 mL working volumes. Each 15-L glass bioreactor was inoculated at a viable cell density ∼2.0 × 105 cells/mL. The Novartis group used two different cultivation strategies for investigation: fed-batch and physiostate operation.
In the fed-batch experiments, a combined glucose/peptone feed pulse was added daily as bolus or given as continuous feed flow. The physiostate cultivations were a specialized form of fed-batch operation. Peptone solutions were applied as with standard fed-batch mode, but defined components such as glucose or amino acids were added to keep their concentration at predefined levels. A process information management system (PIMS) was set to calculate the feed rates based on online HPLC measurements of the limiting metabolites (details below in the Results section).
Equations 1 (left) and 2 (right)

Cell Counting: Daily offline determination of cell numbers was performed with a Vi-Cell cell viability analyzer from Beckman Coulter (www.beckmancoulter.com). This automatic video imaging system is based on the standard Trypan-blue staining method. Measurements of 12 consecutive samples (0.5–1.0 mL) displayed cell density ranges between 5 × 104 and 10 × 106 cells/mL and particle size of 5–70 µm.
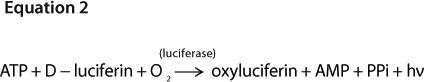
Many methods have been used for ATP determination, including HPLC and electrophoresis (16, 17). Because of its high sensitivity, the most widely used method is the luciferin–luciferase bioluminescent assay (10, 18, 19). During the reaction luciferase converts ATP into AMP (adenosine monophosphate), which produces a flash of yellow-green light with a peak emission at 560 nm. This intensity is achieved under optimum conditions (and at low ATP concentration, to which it is proportionally and linearly related (9, 10), as shown by Equation 2.
Because of its high stability and ease of automation, we chose the ViaLight Plus cell proliferation and cytotoxicity bioassay kit (LT07-221 from Cambrex Bio Science Walkersville). For standard measurements, ATP disodium salt (02055 from Fluka, www.sigmaaldrich.com) was applied.
ATP Analyzer: The ATP Master analyzer is the result of a two-year CTI-funded project between the ZHAW, Novartis, and Biospectra. The basic design of an existing analyzer system (Biospectra’s Glucomaster instrument) was modified to meet the needs of the ATP assay. Biospectra placed a cooling system at the front door of the instrument to keep the assay’s reagent within 4–10 °C. ATP standard solution was placed outside the system, and all lines (e.g., sample and rinse tubing) were connected to the back of it.
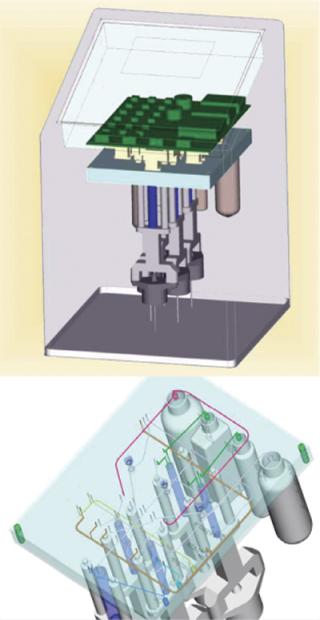
Experiments showed that immediate sample dilution with 1:100 ultrapure water disrupted cell membranes efficiently and inactivated ATPases (data not shown). Furthermore, quenching effects of culture media were also prevented by dilution. A Reglo digital pump was used for taking samples from the bioreactor, its electricity supply triggered by the analyzer. Inactivation and disruption of cells as well as dilution, ATP analysis, and monitoring were automated. To handle small liquid volumes, a novel multilayered steel plate system was developed (Figure 1). And a photo-counting head (H9379 from Hamamatsu, www.hamamatsu.com) was used as a detector.
Results and Discussion
Linearity of ATP Measurement: After evaluating the appropriate kit and conditions for online ATP measurement with the Biospectra analyzer, we performed experiments at the ZHAW focusing on the correlation of growth rate with ATP concentration per cell (Figure 2).

Implementing the ATP-Analyzer into a PAT Workstation: The process analytical technologies (PAT) workstation (WS) is a fully established online monitoring environment for bench-top bioreactors. It consists of online microscopy (OLM) for cell and apoptosis monitoring (20) and online analyzers for medium substrates and metabolites. The rationale behind the advanced monitoring of cell culture processes is to increase process understanding and investigate reaction kinetics of metabolites, which have so far mainly been estimated by modeling and checked against offline analysis of single daily samples.
Other than collecting data for accelerated production, clone selection, and process optimization, the PAT workstation controls all measured variables through a PIMS to preset values during cell cultivation. For data interpretation of most cultivations, information was lacking about the energy balance inside the cells. Because other methods are mainly based on HPLC, the integration of a robust analyzer was necessary with a stable and fully automated analysis method able to automatically calibrate itself. We found the new type of industrial analyzer described here to be stable enough for our requirements.
Online Monitoring of Fed-Batch Cultivations: At the beginning of the cultivation, ATP concentration rose with cell doubling. During growth of all hybridoma cell cultivations, we noticed a sudden decrease in the ATP concentration happening 20–40 hours before decrease in viable cell (VC) concentration. Although the viable cell number still increased, the overall ATP production rate ceased (Figure 5).

Figure 4 shows viable cell density and ATP content of two fed-batch cultivations of SP2/0 cells. Typically an exponential rise in ATP content is correlated with the first days of cultivation until a sudden drop indicates a drift in the central metabolism of production cells to another energy state.

Online Monitoring of Physiostate Cultivations: In most cases, process development led to the need to replenish the essential components cells consumed. In special cases in which physiostate cultivations were used, it was necessary to follow a predefined feed profile by directly measuring the metabolite of interest. The PAT workstation monitored mainly carbon and nitrogen sources in culture media, and we used the data in a software feedback control loop programmed in the PIMS to change the dosage rate of peristaltic pumps dispensing the main nutrients (Figure 3).

As mentioned above in relation to fed-batch cultures, the resulting ATP data also showed a typical pattern (from increase to decrease of concentration in the culture supernatant) in physiostate cultures. By contrast with the fed-batch cultivations, the ATP increase was slower, and the decrease occurred at a later time.
Figure 5 shows that ATP monitoring supplies additional information for feed strategy development. The effective long-term usage of ATP in physiostate cultivations is likely to be based on cell metabolism that’s modified in reaction to the reduced nutrient availability.
Our chosen ATP detection method delivered reliable results after initial set-up adaptations. In theory, cell growth and monoclonal antibody production stops when all essential nutrients are used up and cellular energy levels drop, and the cessation of cell growth should be directly reflected in the ATP level available for more growth. In fact, we observed that ATP production stopped one to two days before maximum cell growth, which is explained by there being enough ATP energy available to start additional cell division cycles before viability started to decrease.

Figure 1:
Identifying the cessation of cell growth earlier in cultivation than with monitoring only cell growth and oxygen uptake enables proactive control of a cultivation’s progress and quality. For process developers, online ATP determination provides a new tool for optimizing processes to achieve longer cell growth times by balancing energy use per cell and by optimizing nutrient supplies to allow for sufficient ATP build-up in cells. ATP concentration remained at higher levels or was available to cells for a longer period with our physiostate cultivations. Experiments involving this cultivation strategy showed higher volumetric growth and cell productivity than were possible with fed-batch culture. The main reason for this seems to be the ATP pool having been partially replenished by use of alternative pathways for glycolysis and amino acid turnover.

Figure 2:
In physiostate cultivations the main nutrients are limited from the start of the cultivation, and the cell-specific ATP content falls quickly whereas cells grow slightly slower than with fed-batch cultivation and initial nutrient excess. After adaptation of the metabolism to alternative pathways, cell growth starts again because energy pools are reloaded with new ATP equivalents. As shown in Figure 6, the ATP/VC peak correlates with the inflection point of the growth curve when exponential becomes linear cell growth. Thus, if the ATP content per cell is high and there is no limiting nutrient supply, then cells will continue to grow exponentially, which is necessary to reach high cell densities in a short time. In some cases, the decrease in ATP/VC could be related to complete consumption of amino acids (data not shown). In other cases, ATP/VC decreased when the amino acids were still available as other substrates such as vitamins possibly became the limiting sources.


A Useful PAT Tool
After a short implementation period, our prototype ATP-Master analyzer was shown to be a valuable and robust industrial instrument with which to detect early changes in cell energy metabolism that are partially reflected in total ATP concentration. The analyzer’s robustness is based on combining a luminescence-based assay with a novel fluid handling system that has fewer moving parts than, for example, online HPLC equipment. After minor modifications, the system was integrated into a PAT workstation environment and has delivered reliable results for different kinds of cultivation strategies.
Online ATP monitoring of cell cultivations in bioreactors has improved process understanding of the build-up of energy equivalents through consumption of nutrients in culture media. From the ATP content of cells, the end of viable cell growth can be predicted one to two days before it occurs. A parallel increase in ATP and cell number leads to optimal cell growth at a constant ATP/VC ratio. In physiostate mode, cells can be successfully cultivated at lower ATP/VC ratios, which reduces the cell growth rate but prolongs cultivation periods and, in most cases, increases product yield per cultivation.
Low levels of ATP/VC were found when cells consumed the substrates from media transferred through inoculation. When the limiting substrate levels were reached, a short adaptation phase of metabolism followed to allow cells to survive with the small amount of nutrients available in a physiostate process. This additional cell energy information provides new opportunities for process developers to optimize cultivation parameters.
In a reproducible process, the same amounts of ATP are achieved by production cells in every new batch run. This makes ATP monitoring a valuable concept for quality control of development processes and inoculum preparation. A high-quality inoculum will not have depleted ATP pools in the cells. If a lower-quality inoculum is used, then ATP content needs to be replenished during production culture, which means a loss of nutrients during the rest of cultivation.
1.) Grammatikos, SI. 1999. Monitoring of Intracellular Ribonucleotide Pools Is a Powerful Tool in the Development and Characterization of Mammalian Cell Culture Processes. Biotechnol. Bioeng. 64:357-367.
2.) al-Rubeai, M. 1992. Specific Monoclonal Antibody Productivity and the Cell Cycle: Comparisons of Batch, Continuous and Perfusion Cultures. Cytotechnol. 9:85-97.
3.) al-Rubeai, M, and AN. Emery. 1993. Flow Cytometry in Animal Culture. Biotechnol. (NY) 11:572-574, 577–579.
4.) Behrendt, U. 1994. Mass Spectrometry: A Tool for On-Line Monitoring of Animal Cell Cultures. Cytotechnol. 14:157-165.
5.) Lovrecz, G, and P. Gray. 1994. Use of On-Line Gas Analysis to Monitor Recombinant Mammalian Cell Cultures. Cytotechnol. 14:167-175.
6.) Renneberg, R. 1991. Enzyme Sensor-FIA-System for On-Line Monitoring of Glucose, Lactate and Glutamine in Animal Cell Cultures. J. Biotechnol. 21:173-185.
7.) Schmid, RD, and W. Kunnecke. 1990. Flow Injection Analysis (FIA) Based on Enzymes or Antibodies: Applications in the Life Sciences. J. Biotechnol. 14:3-31.
8.) Ducommun, P. 2000. A New Method for On-Line Measurement of the Volumetric Oxygen Uptake Rate in Membrane Aerated Animal Cell Cultures. J. Biotechnol. 78:139-147.
9.) Manfredi, G. 2002. Measurements of ATP in Mammalian Cells. Methods 26:317-326.
10.) Crouch, SP. 1993. The Use of ATP Bioluminescence As a Measure of Cell Proliferation and Cytotoxicity. J. Immunol. Methods 160:81-88.
11.) Hardie, DG, and SA. Hawley. 2001. AMP-Activated Protein Kinase: The Energy Charge Hypothesis Revisited. Bioessays 23:1112-1119.
12.) Yang, NC. 2002. A Convenient One-Step Extraction of Cellular ATP Using Boiling Water for the Luciferin–Luciferase Assay of ATP. Analyt. Biochem. 306:323-327.
13.) Atkinson, DE. 1968. The Energy Charge of the Adenylate Pool As a Regulatory Parameter: Interaction with Feedback Modifiers. Biochemistry 7:4030-4034.
14.) Chapman, AG, and DE. Atkinson. 1977. Adenine Nucleotide Concentrations and Turnover Rates: Their Correlation with Biological Activity in Bacteria and Yeast. Adv. Microb. Physiol. 15:253-306.
15.) Chapman, AG, L Fall, and DE. Atkinson. 1971. Adenylate Energy Charge in Escherichia coli During Growth and Starvation. J. Bacteriol. 108:1072-1086.
16.) Buscher, BA, UR Tjaden, and J. van der Greef. 1997. Online Electrodialysis-Capillary Zone Electrophoresis of Adenosine Triphosphate and Inositol Phosphates. J. Chromatogr. 764:135-142.
17.) Theobald, U. 1993. In Vivo Analysis of Glucose-Induced Fast Changes in Yeast Adenine Nucleotide Pool Applying a Rapid Sampling Technique. Analyt. Biochem. 214:31-37.
18.) Higashi, T. 1985. Quantitative and Continuous Analysis of ATP Release from Blood Platelets with Firefly Luciferase Luminescence. Thromb. Haemost. 53:65-69.
19.) Stanley, PE. 1986. Extraction of Adenosine Triphosphate from Microbial and Somatic Cells. Methods Enzymol. 133:14-22.
20.) Lundin, A. 2000. Use of Firefly Luciferase in ATP-Related Assays of Biomass, Enzymes, and Metabolites. Methods Enzymol. 305:346-370.
21.) Bradbury, DA. 2000. Measurement of the ADP:ATP Ratio in Human Leukaemic Cell Lines Can Be Used As an Indicator of Cell Viability, Necrosis and Apoptosis. J. Immunol. Methods 240:79-92.
22.) Garland, JM, and A. Halestrap. 1997. Energy Metabolism During Apoptosis. Bcl-2 Promotes Survival in Hematopoietic Cells Induced to Apoptose By Growth Factor Withdrawal By Stabilizing a Form of Metabolic Arrest. J. Biol. Chem. 272:4680-4688.
23.) Moore, A. 1997. Effects of Temperature Shift on Cell Cycle, Apoptosis and Nucleotide Pools in CHO Cell Batch Cultures. Cytotechnol. 23:47-54.
You May Also Like