Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
November 14, 2016
Sponsored by Cytiva
Single-use pre-sterilized systems can potentially offer a higher level of sterility assurance and a lower risk of product contamination by eliminating some of the operational procedures of traditional processing. The importance of this benefit was highlighted by the FDA’s product recalls in 2015 where 78% of recalls were attributed to lack of sterility assurance or to contamination of the drug product, with the primary reason being failure to follow written procedures.
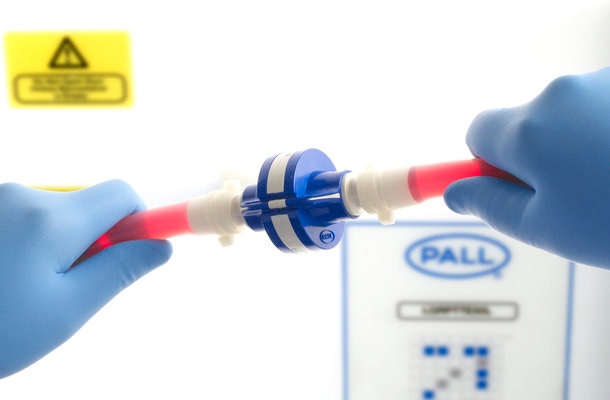
A key requirement for multi-component, single-use sterile systems is the need to make safe and secure connections between the various manifolds and attachments using a sterile connector – and without the need for aseptic handling under laminar airflow in classified environments.
In this white paper, we describe the approach to the design, development and validation of a new sterile connector, the Kleenpak® Presto Sterile Connector. With a unique design and a manufacturing procedure that ensures the robustness, this is the only connector with 100% inspection which is recorded and linked to the individual serial number of each device, giving 100% traceability of every connector. The contribution of this feature to the integrity assurance of single-use systems is discussed as well as other properties of the connector that facilitate the management of sterile connections.
You May Also Like