Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
October 14, 2015
Sponsored by Vironova
Vaccine manufacturers want meaningful information that can be linked to process knowledge to increase product quality and eliminate patient safety issues. New requirements from the United States Pharmacopeial Convention (USP) and the U.S. Food and Drug Administration (FDA) put pressure for root cause analysis to understand impact on changes during process and product development.
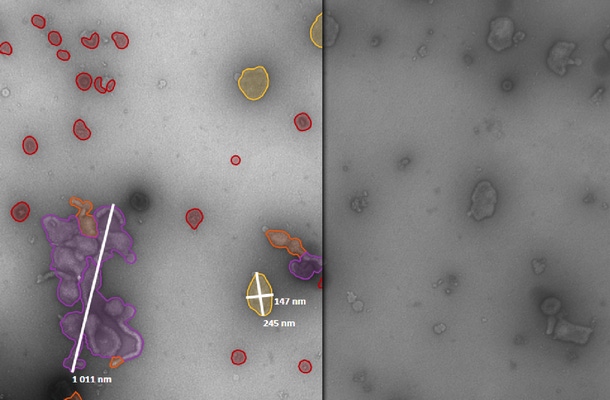
The new demands involve not only size and distribution but deeper characterization that involves morphology and composition studies as part of particle characterization.
Nanoparticles like virus, VLP particles and adjuvants in the range of 0.01-0.1 µm size need to be quantitated, size analyzed and characterized in order to verify particle quality and absence from aggregates or contaminants from the manufacturing process.
This poses the challenge of accurate analytical technologies and direct methods to obtain the morphology data for example in order to distinguish aggregates or particles from debris.
MiniTEM™ is specifically designed for nanoparticle analysis. It is an affordable, automated and easy-to-use solution that covers the measurement range 0.01 to 10 µm. The system delivers particle size distribution as well as morphology analysis data. The low voltage microscope can be placed in any standard office or GMP-compliant laboratory setting.
You May Also Like