Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
September 1, 2008
Several cellular therapies are currently progressing through clinical development with the potential to address unmet medical needs affecting millions of patients. As cell-based therapeutics receive regulatory approval and reach the market, the primary challenge will quickly become manufacturing such products in sufficient volume to meet demand.
Aastrom Biosciences has developed tissue-repair cell (TRC) technology for use in autologous, patient-specific cellular therapy (PSCT) and is conducting late-stage clinical trials both in the United States and Europe. TRCs are derived from a patient’s own bone marrow and processed through a short two-week culture under media perfusion conditions that lend potent functional properties to the final product. Development and clinical use of a PSCT product has raised unique manufacturing challenges that we have addressed through a series of innovative solutions, many of which could have broad application in the field of commercial-scale cell manufacturing.
PRODUCT FOCUS: CELL THERAPIES
PROCESS FOCUS: PROCESSING
WHO SHOULD READ: MANUFACTURING, PRODUCT AND PROCESS DEVELOPMENT, QA/QC, AND PROJECT MANAGERS
KEYWORDS: AUTOLOGOUS CELL THERAPY, SCALE-OUT/SCALE-UP, CELL SEPARATIONS, CELL CULTURE, AUTOMATION
LEVEL: INTERMEDIATE
PSCT has key advantages for patients. In particular, autologous PSCT (treatments derived from a patient’s own cells) has a favorable safety and risk profile not available from allogeneic (universal donor) therapies. Concerns over immune rejection and disease transmission are minimal. In addition, short-term culture reduces the risk of tumorgenic transformation, which is possible in universal donor cell products typically generated from longer-term serially passaged cultures. TRCs have demonstrated a high level of safety in more than 250 patients treated for a variety of medical indications, with both local and systemic administration, showing no sign of cell-related adverse events.
Our TRCs are expanded from cell populations within native bone marrow that are associated with natural cell and tissue homeostasis as well as healing. Local transplantation of these expanded, patient-specific bone marrow cells is expected to facilitate regeneration in patients suffering from damaged or diseased tissues. They are currently under preclinical and clinical evaluation for a range of treatments including vascular, bone, cardiac, and spinal cord tissues.
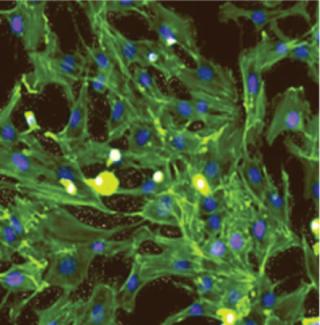
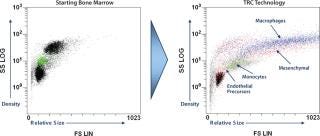
TRCs go through a culture process developed to expand specific subpopulations of cells found within bone marrow, including early stem and progenitor cells, without triggering significant cell differentiation (1, 2). The result is a mixed population of cells rich in those early cell phenotypes. Figure 1 shows representative plots of forward and side scatter generated by flow cytometry to illustrate the distinct morphological differences between TRCs and the bone-marrow mononuclear cell populations they come from. The variety and functions of cell phenotypes are beyond the scope of this article, but the potential benefits of these mixed-cell populations may be found throughout the biological literature.
A Unique Manufacturing Process
Successful commercialization of cell-based, patient-specific products presents a number of unique manufacturing challenges and constraints. Many of these are driven by scale-out (increasing the number of batches) rather than the scale-up (increasing batch size) goal from traditional pharmaceutical or biologics manufacturing.
Risk Management: Of primary importance is the need to appropriately address fundamental risks. The risk that a process will fail to yield a finished cell dose meeting specifications must be extremely low, whatever the reason. For PSCT, manufacturing failure is not merely an operational inefficiency issue; instead, it directly translates into a failed patient treatment. Critical failure rates of most typical cell culture processes are well above an acceptable risk level for PSCT. In addition, the potential of cross-contamination and/or loss of patient identity (chain of custody) for each batch must be maintained at a zero risk level as scale-out occurs and more and more batches are manufactured simultaneously.
Operational challenges also exist because good manufacturing practice (GMP) regulations for conventional drugs and biologics apply to manufactured PSCT products as well (3, 4). Because every cell dose is a separate batch, all requirements for batch records and release must be performed individually for each. Production scheduling can be highly constrained and increasingly complex as scale-out progresses due to the often short shelf-life of both the starting cell inocula and final cell products. Things are further complicated by a need to synchronize manufacture of each dose with the narrow timing needs of a treatment center (e.g., surgery date and time).
“Downstream” Processing: Choices are limited and technically challenging for implementation of postculture finishing steps. Terminal sterilization or filtration of final products is not an option to give the necessary sterility assurance. Typically, dilution is the only option for depleting or clearing unwanted manufacturing materials (e.g., culture media or other culture processing reagents). These issues place increased importance on the quality and safety of raw materials as well as the aseptic processing strategy used throughout manufacturing.
In addition, final cell products must often be formulated into very small volumes (without significant loss of or damage to the cells) to accommodate anatomical constraints associated with some routes of administration. Final-product release testing must satisfy a difficult combination of constraints. Such tests must be rapid (for products with short shelf-lives), relatively inexpensive (because they must be performed for every dose), limited in necessary sample volume (to prevent excessive loss of final product), and able to test a complex product composition. Although many of these postculture processing challenges are common to virtually all cell-based products, they must be innovatively addressed for efficient scale-out of PSCT products.
Scale Issues: Finally, scale-up is certainly desirable wherever possible because of the inherent manufacturing efficiencies of “sharing” a single production batch among multiple patients. But the core steps for manufacturing PSCT products inherently cannot be shared, so other approaches to achieve manufacturing efficiencies must be identified. These may reduce process complexity, labor-saving strategies, computerization, and engineered integration.
Given those challenges, the general drivers that govern the development of a manufacturing process can be quite different for autologous cell therapies. Table 1 indicates how the weighting of some general drivers will differ between a PSCT process and a universal-donor (allogeneic) process.
Table 1: Weighting the drivers for universal and patient-specific process design

TRC Process Summary
Figure 2 shows the end-to-end process Aastrom developed and implemented for the manufacture of TRCs. The series of sequential manufacturing steps begins with aspiration of bone marrow from a patient. This collection of material is not performed at the manufacturing site, and the quality of the material becomes most critical to achieving an acceptable outcome for a well-designed and controlled process. So precise methods and materials (as well as training and certification programs) must be in place to govern its collection.
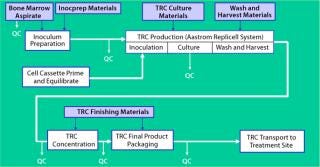
Once that material is received at our manufacturing site, a brief inoculum preparation process is performed to deplete red blood cells from it. The heart of the process is TRC production: inoculation of a culture device with prepared bone marrow, culture-based expansion, washing the resulting cells to deplete culture byproducts that are unsuitable for patient injection (e.g., culture medium and trypsin), and harvest of the TRCs. Once washed and harvested, the cells are concentrated to the final volume required for direct patient administration, then packaged for shipment. Each step is implemented in Aastrom’s proprietary manufacturing technology (Aastrom Replicell System, ARS), a closed automated system developed to support clinical cell production for PSCT. This system is described in detail below as part of a case study illustrating innovative process solutions that address some challenges.
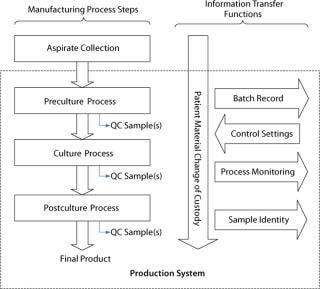
In Figure 3, a generalized process flow for the manufacturing of a PSCT illustrates the need for a series of information-transfer functions to maintain the patient identity of material moving through this process as well as to communicate information throughout. In addition to manufacturing, numerous unique cell therapy challenges are related to shipping logistics, product shelf-life, handling of living products at treatment sites, and administration of cell products to patients. Here we focus on manufacturing, so those will not be addressed.
TRC Culture Process
The core component for TRC production is the ARS Biochamber, which is a type of parallel-plate bioreactor with a liquid compartment in which cells reside and a gas space above that separated by a gas-permeable, liquid-impermeable membrane. The liquid compartment consists of a round plastic disc (the “cell bed”) that is tissue-culture treated to facilitate attachment of adherent cells as well as suitable for gravity settling of nonadherent cells. A liquid layer fills the space between cell bed and gas membrane.
Gas is transported to and from cells by direct diffusion from the gas space through the membrane and liquid layer. Compared with the liquid layer, the membrane has negligible gas-transport resistance. Medium is perfused through the liquid, replenishing nutrients and removing waste products (Figure 5). Because the required rapid gas transport is decoupled from the slower nutrient and metabolite transport, a slow medium perfusion rate can be used such that shear stress is negligible and secreted growth factors in the cellular microenvironment are retained while low–molecular-weight metabolic by-products (e.g., lactate) are removed from the cell bed. For culture of bone marrow, this has provided a tissue-like environment of stromal and other accessory cells, stem cells, progenitors, and mature cells. Figure 4 is a cross-sectional illustration of the transport dynamics.
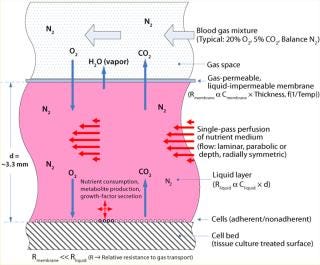
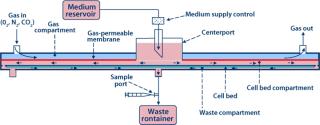
To implement the perfusion function, medium is introduced at the center of the Biochamber’s liquid compartment (or cell bed compartment). The medium radially flows toward the perimeter of the Biochamber, passes through a series of holes spaced around that perimeter, flows radially back toward the center through a thin layer underneath the cell bed (waste compartment), and exits through a waste port at the center. Because oxygenation is not part of it, enabling a slow perfusion rate, even fully nonadherent cells won’t be displaced by the flow, so recirculation is not required. In addition, radial flow eliminates nonhomogeneous “wall effects” that occur in other parallel-plate bioreactor geometries with flow parallel to their walls. Referred to as single-pass perfusion, this unique operating mode is proprietary to Aastrom Biosciences.
In addition to providing an effective biological process, the Biochamber provides several other advantages:
Lower process complexity than other bioreactor designs (e.g., no recirculating pump, external oxygenator, or sparging device)
Hydraulically filled, sterilely closed culture chamber that directly facilitates incorporation into automated, closed-system technology
Open culture space allows cell harvest with high recovery and viability
Robust culture in the instance of control-system failure (e.g. perfusion, gas exchange)
Controlled separation of fluids is enabled without dislodging or damaging cells.
The primary trade-off for this design is that it is a fixed-scale device with a relatively low dimensional efficiency. For PSCT, however, scale-out is the core need, so this trade-off is acceptable.
The Biochamber is incorporated into a single-use, disposable ARS Cell Cassette (Figure 6), which also includes a preconnected set of flow-path elements and reservoirs to provide all necessary functions to support a self-contained, sterilely closed culture process. The Cassette further includes features for interface with our ARS instrumentation platform to implement significant automation from initial priming of the Biochamber through to final harvest of cultured cells. Sterile docking with a commercially available tube welder connects the Cassette to containers for reagents, inoculum, and collection required before and after a culture process. So this process can be performed in a minimal class 100,000 (about Grade D) cleanroom while maintaining the necessary sterility assurance.
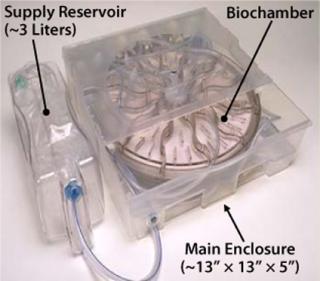
Integrating Process Steps: An Innovative Solution
Traditional approaches for cell culture harvesting produce cells mixed with culture byproducts (e.g., culture media and harvest reagents). Those need to be separated or washed away in an additional processing step before cells can be acceptable for patient administration. For adherent cell cultures, we can drain away the culture medium and rinse the cells before harvest (resulting in no cell loss). But an enzyme such as trypsin is required to release those adherent cells from their culture surface, then must be separated from the collected cells.
For nonadherent cell cultures no enzymes are required, but the culture medium must be separated from collected cells. For mixed cultures of adherent and nonadherent cells, such as our TRCs, culture medium carrying the nonadherent cells is first collected before a harvest enzyme is used to release the adherent cells from their culture surface. The resulting enzyme-containing cell solution is combined with the previously harvested nonadherent cells, and a downstream separation is used to deplete both harvest reagent and media components.
There are existing methods and equipment for performing this separation step in clinical use. However, most can cause significant cellular damage (resulting in loss of viability and biological function) by mechanical forces applied during the separation process. Significant loss of cells can occur during transfer steps into and away from a separation apparatus. In the case of a mixed cell population, the process can even cause a shift in cell profile because of preferential loss of larger, more fragile subpopulations.
Separation methods for washing cells are commonly based on centrifugation or filtration. Several systems, some fully automated and sterilely closed, are commercially available for each method. For example, the Cytomate cell washer (from Baxter International Inc. of Deerfield, IL) incorporates an automated tangential-flow filtration process using a spinning filter membrane and the COBE 2991 cell Processor (COBE BCT, Lakewood, CO) implements a semi-automated, centrifugation process. Both have been used in TRC processing. In each case, however, the process exposes cells to mechanical shear and typically causes significant loss in cell viability as well as subsequent population shift. Furthermore, cell yield is compromised by the multiple transfer steps required.
To address those issues, Aastrom developed a novel, integrated, wash-then-harvest technique for its TRC process, in which cells are washed within the Biochamber before collection. Liquid displacement techniques made possible by the Biochamber design allow for nonadherent cells to be separated from their suspension medium while remaining in the Biochamber. This technique removes two cell transfer steps, both to and from a separate cell-washing device, and increases the corresponding cell yield. Since the cells are exposed to only liquid displacement with low mechanical stress, their resulting viability is both higher and more consistent than when they’re washed in a separate device. This provides a harvested cell suspension that is immediately ready for final volume reduction and suitable for patient administration, greatly simplifying final processing steps while increasing the quality of the final product. Finally, the need for additional capital equipment and associated disposable supplies has been eliminated.
TRC Manufacturing Automation
Automation is a powerful tool and absolutely essential for efficient commercial manufacture of PSCT products. However, it can be inappropriately applied and/or over-used. In fact, all other considerations being equal, automation increases complexity, driving up costs and failure rates. The rationale for automation depends on each specific situation. Table 2 summarizes some qualitative rationale that guided our decision-making in relation to the ARS. Automation is a term that can have as many meanings as there are people using it. To help clarify, we describe various types of automation incorporated into the ARS: process automation, procedural sequence automation, and information automation.
Table 2: Qualitative rationale for ARS automation development

Process automation refers to automated systems typically used for providing steady-state control of operation parameters within bioreactor systems (e.g., flows, temperatures, pressures, and so on). For the ARS, each Cell Cassette is placed into a dedicated ARS Incubator that automatically controls all process functions including perfusion rate, gas delivery (mixed from pure N2, O2, CO2 to any blend), incubation temperature for the Biochamber, and refrigeration temperature for the integral medium supply reservoir. To achieve a very high mission-critical success rate, the Incubator also incorporates an extensive monitoring system to confirm that all process functions are operating correctly and, if necessary, to automatically place a process into a “safe state” if an equipment failure is detected. To help ensure that the monitoring system comprehensively covers all reasonably possible failure modes, a failure mode and effects analysis (FMEA) is performed to methodically chart each failure, its effect, detection method, and mitigation.
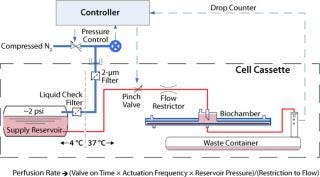
The summary schematic in Figure 7 shows the perfusion control system. As described, the Biochamber design allows for a simplified single-pass perfusion of medium. As a result, this subsystem offers low mechanical and interface complexity as well as being low in cost. Additionally, the design is inherently safe, both in regards to the risk of excessive pressure build-up and in sterility assurance, when compared with other fluid-pumping approaches. A drop counter placed downstream of the culture process monitors flow rate based on the volume of each drop, which provides for comprehensive detection of control system problems (e.g., obstructions, leaks, and so on).
Procedural sequence automation refers to the use of robotics, automated sequencing equipment, or other types of devices that perform a sequenced set of steps to complete some type of procedure, such as harvesting cells from a bioreactor. For the ARS, each Cell Cassette is loaded into the ARS Processor for a short period (30–90 minutes), applicable reagent supply and/or collection containers are sterile docked, and automated sequences of steps are performed from a menu of tables that define valve actuations, robotic Cassette movements, and various control and monitoring criteria. For TRC production, the Processor is used before culture to prime and inoculate a Cassette, ensuring that the key requirements for starting the culture are met: The flow path is fully primed with medium, cells are uniformly distributed throughout the cell-bed surface area, and the Biochamber liquid space is completely filled and purged of all air. The Processor is used again at the end of each process to automatically wash and harvest cells from the Biochamber, ensuring that culture materials have been depleted and that a high percentage of cells have been successfully collected in a controlled volume. The flow chart in Figure 8 illustrates the general use of the Processor for procedural sequence automation.
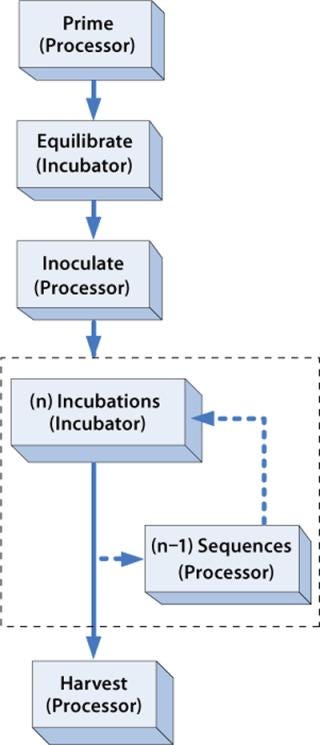
Information automation addresses the information-transfer requirements represented in Figure 3. Manufacturing execution systems (MESs), radio-frequency identification (RFID), bar-coding, and networks are some components used to implement this type of automation. For the ARS, the central component of the information strategy is the ARS Application Key, which is an electronic read–write memory device (similar in function to an RFID tag) that is initialized and attached to each Cassette at the start of a process. It is programmed with Incubator operating parameters (e.g., perfusion schedule and gas mixture over time), Processor sequence selections, and batch identification. When a Cassette is inserted into an instrument, it automatically reads the information on the Key and executes accordingly. Each instrument also writes process events onto the Key (e.g., start and end of process steps, alarm events) to support automated generation of batch-record information. The instruments continually update status to the Keys, too, so that each time a Cassette is inserted into one or power is restored after an interruption, it knows exactly what point in the process a Cassette has reached. The Key also contains a real-time clock to provide a precise time index at all points.
Factory Automation: The ARS has been used to provide automated cell production for numerous clinical cell therapy applications, first for point-of-care cell processing and more recently in centralized manufacturing. As regulations for cell therapy products have evolved over the past decade or so — e.g., the FDA/CBER definition and interpretation of “more than minimally manipulated” products (5, 6), practicality has, in many cases forced a migration from hospital-based cell processing to centralized manufacturing facilities targeting a biologics license application (BLA) or its equivalent. As a result, a fourth type of automation presents significant opportunity to increase manufacturing efficiency: We call it factory automation. This level of automation would, for instance, facilitate material handling and movement of Cassettes through a high-throughput manufacturing facility. Although many companies (including Aastrom) have not yet reached a scale that would justify such systems, they will need to be planned and implemented as PSCT products move closer to BLA approval and commercial launch.
Future Challenges
As clinical trials progress to larger cohorts, and cell therapy products obtain marketing approval, new challenges will be identified. This will lead to the need for novel scale-out strategies and, wherever and whenever possible, scale-up solutions that efficiently manufacture PSCT products under high-throughput conditions — while continuing to meet requirements for safety, consistency, and reliability.
Although inherently limited for PSCT, opportunities for scale-up rather than scale-out represent the most attractive choices to realize economies of scale. They include all requirements that are not specific to a particular batch of cells: management and control of culture feeds (e.g., medium, gases, processing reagents), incubation environment (e.g., temperature, humidity), and facilities and waste management. Scale-up of controls for raw materials and product specimens will strain higher throughput operations, creating the need for new information technology infrastructure and automated data management implementation to support inventory and product control. For example, inventory control and patient-specific product tracking will be necessary to maintain a chain of custody and ensure that each patient is treated with his or her own cells.
With scale-up opportunities identified, scale-out must then be addressed for all batch-specific steps that cannot be shared: inoculum preparation, culture, harvest, washing, volume reduction, final formulation, and packaging. By comparison with universal allogeneic therapies, with which scale-out is typically not required until individual doses are prepared at the end of a process (e.g., final fill, cryopreservation), the need for scale-out is much more extensive for PSCT products. Simplifying and integrating steps provides significant benefits. The integration of wash and harvest steps described herein is a prime example. A future possibility is the integration of automated online sampling and/or quality control (QC) measurement into manufacturing processes, which could include reduced labor and processing times while providing greater consistency.
Adapting automated solutions from other industries will accelerate progress. For instance, culture-based protein bioprocessing (e.g., antibody production) uses some technologies and approaches that can potentially be leveraged, as do specimen management tools used to build libraries for drug discovery, directed donations managed by blood banks, and made-to-order manufacturing operations. We could borrow automated solutions already in place in high-throughput screening laboratories at many pharmaceutical companies for incorporation into automated QC. High-throughput screening and associated multiplexing technologies that have been developed to address their challenges can be directly applied to high-throughput PSCT production.
All scale-up or scale-out activities will need to undergo a cost-benefit analysis. The breadth of technologies and scope of development necessary for these purposes will require significant capital expenditure, and small cell therapy companies may not have sufficient cash to invest in long-term scaling needs before their market is proven. The cell therapy industry would benefit from establishing standards for much of the shared technology that is needed, but competitive pressures will present a significant barrier to overcome before such benefits can be realized. Most likely, standardization will be driven by industry consolidation and/or substantial investment in infrastructure by well-capitalized product and service providers — or partners who can combined expertise in PSCT, scale-out and scale-up bioproduction, and automation.
1.) Schwartz, R, B Palsson, and S. Emerson. 1991. Rapid Medium Perfusion Rate Significantly Increases the Productivity and Longevity of Human Bone Marrow Cultures. PNAS 16:155-162.
2.) Mandalam, R, M Koller, and A. Smith Schindhelm, K and R. 1999.Ex Vivo Hematopoietic Cell Expansion for Bone Marrow TransplantationEx Vivo Cell Therapy, Academic Press, San Diego:273-291.
3.). Current Good Manufacturing Practice in Manufacturing, Processing, Packing, or Holding of Drugs: General.
4.). Current Good Manufacturing Practice for Finished Pharmaceuticals.
5.). Proposed Approach to Regulation of Cellular and Tissue-Based Products.
6.). Human Cells, Tissues, and Cellular and Tissue-Based Products.
7.) Au, P. 2008. Bone Marrow Derived Mesenchymal Stem Cells Facilitate Engineering of Long-Lasting Functional Vasculature. Blood epub ahead of print.
8.) Dennis, JE. 2007. Clinical-Scale Expansion of a Mixed Population of Bone Marrow-Derived Stem and Progenitor Cells for Potential Use in Bone Tissue Regeneration. StemCells 25:2575-2582.
9.) Koike, N. 2004. Tissue Engineering: Creation of Long-Lasting Blood Vessels. Nature 428:138-139.
You May Also Like