Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
People who regularly culture animal cells become so comfortable with standard techniques that novel approaches can seem contrived or even unnatural. However, the typical cycle of seeding cells at very low density in an excess of medium and harvesting (often quite aggressively) just before the point of medium exhaustion is quite an unphysiologic process. Popular culture systems often take cells that originally grew attached to a porous matrix at high densities, with little variability in nutrient and oxygen supply, and adapt them to low-density, styrene-bound or amorphous suspension cultures. Although these methods are well understood and convenient, classical batch-style two-dimensional culture in T-flasks or three-dimensional suspension culture in shake-flasks and bioreactors really aren’t physiologically relevant models.
A remarkable number of alternative culture approaches operating with such unusual mechanisms as rocking bags and depth filters have been introduced over recent years. These are often categorized by characteristics such as suspension/adherent or batch/continuous operation (1). However, most share two fundamental features: Cells are subjected to wide swings in nutrient, waste, and pH levels from seeding to harvest, and they are generally growing at highly cyclical but relatively low culture densities.
PRODUCT FOCUS: CELL CULTURE PRODUCTS
PROCESS FOCUS: PRODUCTION, PRODUCT DEVELOPMENT, UPSTREAM PROCESSING
WHO SHOULD READ: MANUFACTURING AND PROCESS ENGINEERS, ANALYTICAL LABORATORY PERSONNEL
KEYWORDS: PERFUSION CULTURE, MEDIA, VACCINES, BIOANALYSIS, CELL THERAPY
LEVEL: INTERMEDIATE
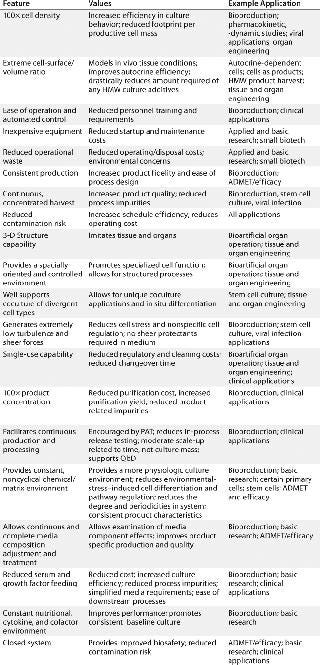
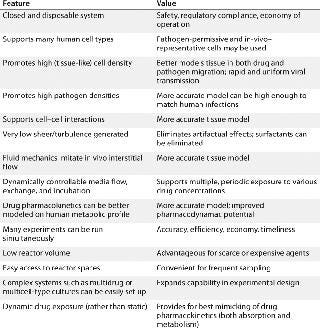
Until recently, the negative consequences of those highly artificial protocols were not well appreciated. But increasing demands of modern drug development, regenerative medicine, and fundamental scientific investigation have inspired the development of alternative approaches. Perfusion culture now exists in a number of (often quite distinct) implementations (2). Hollow-fiber–based technologies in general are used in many applications, from tangential-flow filtration to prokaryotic biofilms in wastewater treatment. Here we consider only their use in the culture of animal cells, referring to this as hollow-fiber perfusion bioreactor (HFPB) technology. Table 1 introduces many of the growing applications of such systems along with the benefits they provide.
Table 1: Features, values, and example applications of hollow-fiber perfusion culture
The Basics of Hollow-Fiber Perfusion
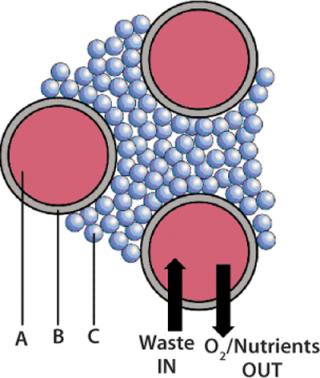
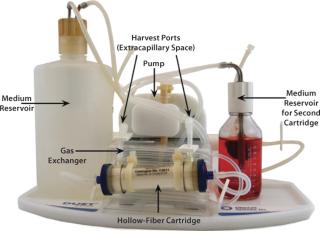
HFPB is a high-density, continuous perfusion culture system. Its hallmark component is a set of thousands of semipermeable hollow fibers in parallel array within a tubular housing or cartridge that’s fitted with inlet and outlet ports. The fiber bundles are systematically potted (attached) at each end so that any liquid entering a cartridge will necessarily flow through their interior. Animal cells are generally seeded within a cartridge but outside the hollow fibers in what is referred to as the extracapillary space (ECS) (Figure 1). Culture medium is pumped through the lumen of the hollow fibers, allowing nutrients and metabolic products to diffuse both ways across the fiber walls. Having passed through the cartridge, medium can be either oxygenated and returned to it or collected while fresh medium is introduced (Figure 2).
A wide range of materials — e.g., polysulfone and cellulose derivatives — can be used for the hollow fibers. Molecular weight cutoffs begin at 5 kDa and go up to virtually any desired upper limit. The fiber materials can vary in such properties as percent porosity, molecular weight cut-off, and hydrophilicity, and they can be further modified during either manufacturing or their actual application to introduce defined functionalities onto their surfaces. Three fundamental characteristics of an HFPB system are
extremely high binding culture surface to volume ratios
immobilization of cells at a very high (biomimetic) density on a porous matrix supporting prolonged culture
selectable porosity of the fibers for such purposes as concentration of secreted product.
History of HFPB: The first success in hollow-fiber culture demonstrated that such an approach was feasible and suggested some benefits that have since become better understood (3). Shortly thereafter, commercially available systems were presented by such companies as Amicon (now part of Millipore Corporation, www.millipore.com/amicon) and Endotronics (now Biovest International, www.biovest.com), and they demonstrated some utility in the unique potential HFPBs afford. However, those early systems were limited by their less-capable media pumps and fiber materials such as cuprammonium rayon and cellulose acetate, which offered poor gross filtration rates. In the protein production arena, that made them sufficient for hybridoma cultures at limited cell densities, but early units had insufficient nutrient and waste exchange to support the extended culture of such increasingly popular expression lines as CHO and HEK293.
Large-scale cell culture depends on the mass transfer rates of poorly soluble oxygen, which was the Achilles’ heel of earlier attempts at larger-scale hollow-fiber systems. Engineering advances and newer materials are now addressing those limitations: Newer, large-scale hollow-fiber bioreactor systems are being designed with ECS ≤ 1 L (4). In the past few years, system advances and increased demand for the unique features provided have resulted in a resurgence of interest in HFPB.
Features and Benefits
In most culture systems, cells are seeded in a medium containing a great excess of nutrients and no metabolic products. They progress for a matter of days in an environment of declining nutrients and increasing products, only to be suddenly exposed to the original media composition again (when the culture is split into fresh media) or to a slightly different variation of the original formulation (during serial adaptation of a culture to a new medium). Recent advances in metabolic flux analysis support the significance of such exposure to a variable and even discontinuous cycle of nutrient and metabolic products (5). However, the relative constancy of the culture matrix and ambient chemical environment in an HFPB system provides a more consistent and physiologic culture environment. Because freshly oxygenated medium is continually exchanged through an immobilized culture, cells exist in an environment of relatively constant metabolite and growth-factor concentration. This benefits a number of research, modeling, and production applications. For example, some primary and specialized cell lines tend to regulate critical pathways, differentiate, or “shock” in response to significant or sudden changes in their medium.
The HFPB culture chamber environment is continuously controllable in real time. Because HFPB systems possess such an efficient medium-exchange mechanism, it is easy to alter the input medium composition (and therefore the ambient cellular environment) whenever desired. This differs from fed-batch cultures, which allow only the addition of a bolus of nutrients or reagents on top of existing media components. Furthermore, high-density culture in the controlled hydrodynamic conditions of an HFPB can provide a microenvironment of directional flow, establishing a gentle interstitial gradient within the cell mass for autocrine stimulation, cell alignment, and desirable cell–cell or cell–surface interactions.
Because an HFPB cell culture (on the ECS side of the fibers) exists at concentrations ≥100× that of standard suspension cultures, it was discovered early on that less serum would be required and product could be harvested at many times the concentration of that from most other systems. More benefits are being identified now, including facilitation of adapting cultures to serum-free media and better support for conditioned media and autocrine-dependent cultures. The initial volume of a circulating loop can be very low when a culture is seeded, then raised as the cell number increases, thereby maintaining a more constant cell/medium ratio.
Cells in HFPB culture are separated from the bulk of their medium by a membrane of definable composition and porosity. They essentially experience two different volumes: That seen for low–molecular-weight components such as glucose and lactate will be relatively large, whereas the volume seen for larger components and some stimulatory cytokines will be ≥100× smaller. Because both the culture (ECS) and perfused medium (fiber lumen) sides of the system are accessible to sampling and feeding, it is common to maintain and monitor particular components within each distinct space. This provides for many valuable functions in operation.
For example, the volume of the cell-containing compartment can be quite small, so products can be harvested at 100× or higher concentration than from suspension culture. By matching a reactor’s fiber porosity to cell characteristics, products may be accumulated, maintained, and measured on either side of the system. In one application, macromolecular culture factors can be introduced or allowed to accumulate within the ECS side. So for instances in which secreted factors either stimulate or inhibit growth, their effects often can be modulated by selection of the fibers’ pore size, such as allowing TGF-β or TNF-α to diffuse out and be efficiently pumped away.
Combining such features as an unlimited nutrient supply and the ability to “debulk” a culture through the cartridge ports allows an HFPB system to be maintained at relative equilibrium for several months or longer. Continuous production over long periods provides several benefits over batch cultures: consistency in culture condition, dramatically increased production per unit footprint and culture volume, continuous or daily product harvest that allows timely stabilizing treatment or storage conditions, and the option of continually removing products from a culture that might be toxic or inhibitory to cells. A feature almost uniquely provided by HFPB systems is long-term support of divergent cell types in coculture at even extreme ratios.
Applications
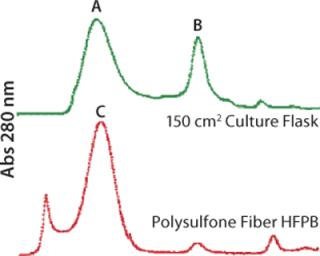
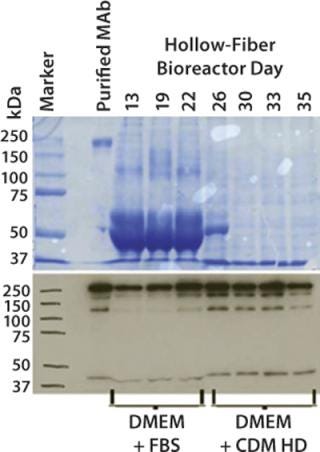
Significant improvements in HFPB systems are supporting the demand for production of high–molecular-weight and highly processed proteins (e.g., MAbs) in such cell lines as hybridomas, CHO, HEK293 and HepG2. High cell densities and continuous nutrition gives dramatically higher product harvest concentrations as well as higher and more consistent product quality (Figure 3). Recently, the availability of chemically defined and animal-product–free media formulations specifically designed for high-density perfusion applications has further promoted the use of HFPBs in the production of secreted products (Figure 4) (6). Use of hollow-fiber bioreactors for protein biological production can profoundly affect downstream processes, from reducing media-volume–related total endotoxins to easing initial purification steps.
Vaccines: The field of human vaccine design and production is in the midst of revolution. Classical vaccines for influenza are transitioning to cell-culture–based manufacturing. Beyond that, a number of entirely new approaches to vaccination involve new materials and production technologies. In cell-culture–based production of whole-virus, subunit, or virus-like– particle vaccines, HFPB features of rapid establishment and turn-around, operation as a closed and disposable system, reduced harvest volumes, and extended culture capability are of particular importance. It seems that culture conditions can affect the fidelity of virus-like particle formation differently from more commonly secreted products.
The first feature supporting HFPB use as a virus production platform is the ability to culture many different and specialized cell types. Low-MOI (multiplicity-of-infection) infections can benefit from the extreme cell density and controlled media flow, which promote progression of infection through a culture. Cultures with advanced infections benefit from extremely low shear forces. Success has been reported with a wide range of virus types and culture systems including hepatitis C, Epstein-Barr virus, baculovirus, Vaccinia, and human immunodeficiency virus (7). Applications range from basic research to viral product manufacturing.
Bioanalytical Uses: The number of quite distinct in vitro models applied to absorption–distribution–metabolism–excretion–toxicology (ADMET) and efficacy studies in cosmetics and pharmaceutical development is growing rapidly. Many systems are either in advanced stages of development or in actual application because a scarcity of regulatory or licensing issues here makes the use of novel, well-controlled, and science-based systems compelling. HFPBs offer the best control of complex, and high-density growth, infection, treatment, and serial sampling regimens. They permit a more realistic simulation of in vivo drug effects in a dynamically controlled system that more accurately than other systems models such activities as bioavailability and diffusion (Table 2, Figure 5) (8).
Table 2: Advantages to HFPB for in vitro pharmacokinetic and -dynamic modeling
In viral pathology modeling, HFPB units can be charged with viruses or virally infected cells in an excess of noninfected cells. The progression of infection through an immobilized 3-D and even cocultured cell mass well models its progression in actual animal tissue. Comparison of results from this system with in vivo infections show that the extremely high cell densities, continuity of ambient nutrition, and continual perfusion allow this system to faithfully replicate many aspects of in vivo infections. Various animal species, tissues, and viruses can be modeled (9).
A related application of HFPBs is the pharmacodynamic/kinetic modeling of antiviral compounds. Beginning with the above model of virus pathology, computer-controlled pumps can expose multiple units to candidate antiviral compounds, even delivered at differing concentrations and intervals to simulate known human pharmacokinetic profiles. A useful feature of this system is the ability to perfuse drug at a constant rate through the intracapillary space (allowing baseline modeling) or to periodically introduce drug directly into the cell-mass–containing ECS to better imitate the intermittent nature of dosing in clinical patients (10).
Cell/Tissue Therapy: Although adoptive immunotherapy has provided significant promise for decades, clinical results have indicated a need for both immunological and process system improvements. HFPBs have been used to improve such cell-based therapies by, for example, dramatically reducing both the cost of stimulation and the volume of stimulated cells to be processed for reinfusion to a patient (11).
In bioartificial organ research, the structural similarity of an HFPB to many aspects of an animal organ makes it a natural candidate to supply organ functions in clinical applications. Like an animal organ, an HFPB contains an immobilized 3-D mass of cells continually and sterilely perfused through a microporous scaffold assembly. By charging a reactor ECS with cell types that provide elements of organ function, then perfusing a patient’s blood through the intracapillary space, an HFPB can modify plasma in ways an organ would (12).
The many physical and functional similarities of HFPBs to animal organs also allow them to be effective surrogates for animal systems in tissue and organ engineering that is, the in vitro generation of a cell mass to imitate actual organ tissue. Here especially, fluid movement within a cell mass imitating in vivo arterial or venous hemodynamics is a great advantage. HFPB hydrodynamics define a subtle asymmetry across the cell mass, contributing to a biomimetic tissue-like structuring and orientation of cells within it. By charging the ECS with organ cells or their precursors, then perfusing with media containing appropriate growth factors, 3-D cell masses with tissue-like properties can be produced.
Stem Cells: Features particularly supporting stem cell culture are high culture densities, low turbulence and shear, a constant nutritional environment, and the fact that matrix materials such as hydrogels may be infused within an HFPB cartridge during culture. Also, the surface of hollow fibers may be functionalized with receptor-specific ligands such as antibodies to specifically select particular cell types, or with extracellular matrix factors to provide a more in-vivo–like surface for cell attachment.
Improvements in HFPB materials and engineering (such as the emergence of larger-scale systems) inspire a growing interest in using HFPBs for such established applications as the production of protein biologicals. And the continuing development of biotechnological systems themselves, from in vitro modeling to bioartificial organs, is creating new demands for some of HFPB unique features. The recent H1N1 pandemic emphasizes the value of very recent applications in influenza antiviral pharmacodynamics (13).
1.) Jain, E, and A. Kumar. 2008. Upstream Processes in Antibody Production: Evaluation of Critical Parameters. Biotechnol. Adv. 26:46-72.
2.) Li, L. 2009. A Single-Use, Scalable Perfusion Bioreactor System. BioProcess Int. 7:46-54.
3.) Knazek, R. 1972. Cell Culture on Artificial Capillaries: An Approach to Tissue Growth In Vitro. Science 6:65-67.
4.) Cadwell, JS.
5.) Zhou, S, Z Cui, and JPG. Urban. 2008. Nutrient Gradients in Engineered Cartilage: Metabolic Kinetics Measurement and Mass Transfer Modeling. Biotechnol. Bioeng. 101:408-421.
6.) CDM-HD Serum Replacement: Chemically Defined Medium for High Density Cell Culture, FiberCell Systems, Frederick.
7.) Leong, M. 2007. A Hollow-fiber Bioreactor for Expanding HIV-1 in Human Lymphocytes Used in Preparing an Inactivated Vaccine Candidate. Biologicals. 35:227-233.
8.) Deziel, M. 2005. Identification of Effective Antimicrobial Regimens for Use in Humans for the Therapy of Bacillus anthracis Infections and Postexposure Prophylaxis. Antimicrob. Agents Chemother. 49:5099-5106.
9.) Beauchemin, CAA. 2008. Modeling Amantadine Treatment of Influenza A Virus In Vitro. J. Theoret. Biol. 254:439-451.
10.) McSharry, JJ. 2009. Pharmacodynamics of Cidofovir for Vaccinia Virus Infection in an In Vitro Hollow-Fiber Infection Model System. Antimicrob. Agents Chemother. 53:129-135.
11.) Malone, CC. 2001. Characterization of Human Tumor-Infiltrating Lymphocytes Expanded in Hollow-Fiber Bioreactors for Immunotherapy of Cancer. Cancer Biother. Radiopharmaceut. 16:381-390.
12.) Silva, AI, and M. Mateus. 2009. Development of a Polysulfone Hollow Fiber Vascular Bioartificial Pancreas Device for In Vitro Studies. J. Biotechnology. 139:236-249.
13.) McSharry, JJ. 2009. Prediction of the Pharmacodynamically Linked Variable of Oseltamivir Carboxylate for Influenza A Virus Using an In Vitro Hollow Fiber Infection Model System. Antimicrob. Agents Chemother. 53:2375-2381.
You May Also Like