Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
Sampling is used extensively to monitor both behavior and quality throughout biopharmaceutical processesing (1, 2). Methods must deliver representative samples and — more important — not compromise the integrity of a given unit operation or the process of which it is part. When microorganisms, animal cells, viruses, or nonfilterable materials are involved, sampling methods must not introduce contamination (see the “Regulatory Requirements” box). For successful sampling, three methods have been used routinely over the years: steam-in-place (SIP) valves; aseptic tube welding; and single-use, presterilized sampling devices.
Each method has its advantages and limitations. When a source of steam cannot or should not be brought to a small and mobile stainless steel vessel, a commonly used method is full autoclaving, in which the entire vessel is placed in a large autoclave for sterilization. Tube welding by heat is a method that remains in use and could be considered for a fully autoclaved vessel; however, it has significant constraints and limitations due to requirements for qualified equipment and trained personnel.
In the biopharmaceutical industry, adoption of single-use methods for sampling from stainless steel equipment has demonstrated measurable benefits over traditional steam-in-place valves and tube welding methods. Closed, single-use sampling devices that are compatible with autoclave use offer advantages over other methods because they are preattached to a given vessel, are very easy to operate, and require no extra equipment. The most significant benefits are virtual elimination of cross-contamination risk, decreased labor requirements, and reduced time required for sampling (and between samples). It usually takes about an hour to get a sample using a steam-in-place valve; by comparison, the time to complete a sampling sequence using a preinstalled disposable sampling device is one to two minutes.
Photo 1:

Photo 2:

Despite the advantages offered by single-use sample devices, however, autoclaving them can present a challenge (3, 4). Autoclave cycles involve high temperatures, repeated and rapid pressure changes, and varying cycle periods. Materials of construction must be compatible with irradiation. Here we describe how those challenges can be overcome and discuss a robust solution for sampling with single-use devices for demanding autoclave applications.
When subjected to sterilization by autoclave, a single-use sampling device must meet the following requirements in a complex balancing act:
Be compatible with a presterilization method such as gamma or beta radiation or ethylene oxide
Meet requirements for leachables and extractables
Allow venting without compromising integrity
Minimize condensation inside.
So the autoclaving process must not affect the representation of the sample that will be collected.
Successful design and development of single-use sampling devices requires a thorough understanding of the behavior of materials — plastics, in particular — in response to large variations of temperature and pressure during autoclave cycles. Robust testing is then required to validate desired performance.
Autoclave Basics
Autoclaving is a process that uses steam as a sterilizing agent under specific pressure and temperature conditions. This process depends on the relationship between two key parameters: time and temperature.
Standard temperature and pressure relationships for autoclaving include 121 °C and 1 bar (250 °F, 15 psi) and 134 °C and 2 bar (273 °F, 30 psi). Duration of an autoclaving cycle can be adjusted according to load type, size, and density. The probability of a nonsterile unit decreases with longer cycle times and higher temperatures.
Each autoclave procedure must be validated before it is put into routine use. The nature of the materials involved, as well as the shape, mass, and cleanliness of what is placed into an autoclave will directly influence the sequence of events that ultimately determines sterilization. One of the most critical aspects is to identify what part of a given load is most difficult to sterilize. That will determine the overall autoclave cycle parameters. Thus, the rest of the load must withstand those parameters.
In most cases, the combined complexity, heat capacity, and conductivity of stainless steel components drive their requirements for heat and circulation of steam. Thus, any subpart of such a load that is made of plastic must be designed to withstand exposure to temperatures of ≤134 °C and be vented to prevent excessive stress that could compromise device integrity due to forces resulting from pressure differences.
Below, we describe three basic types of autoclave cycles. Characterization of a load will determine the type of profile to be used. Subsequently, cycle validation will fully define the cycle parameters.
Hard Goods: A typical hard-goods cycle (Figure 1, TOP) begins with a vacuum phase before steam injection. It allows the desired sterilization temperature to be achieved and removes insulating air that retards heat transfer. That initiates a sterilization dwell period, at the end of which pressure decreases to be equilibrated to atmospheric. This cycle is suitable for components that are easy to sterilize, with a design that allows for easy steam circulation. For example, many types of glassware and compartments offer a favorable ratio of volume to opening.
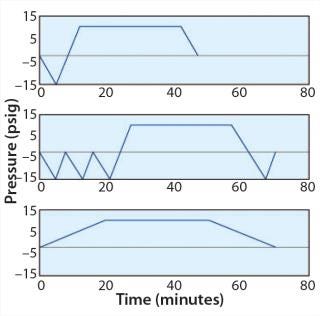
Wrapped Goods: A typical wrapped-goods cycle (Figure 1, MIDDLE) includes three or more vacuum cycles before steam
injection. To eliminate condensation of liquid water, a sterilization vacuum is required after sterilization to generate a flow that helps water exit the compartments. Steps before and after sterilization are often longer than the sterilization dwell period itself. This type of cycle is typically used for long lengths of tubing and vessels with small openings.
Liquids: For a liquids cycle (Figure 1, BOTTOM), if a vacuum cannot is created too rapidly, then liquid will be drawn out of its container. So a slow exhaust is used to prevent that. The cycle duration is longer when the volume of liquid to sterilize is large because the time it will take to reach the appropriate sterilization temperature is longer. If a cooling step is required after the dwell period, then the cycle duration may be considerable. Such a cycle is typically used for containers that hold fluids.
For full autoclaving of an entire stainless steel vessel, in most cases the basic wrapped-goods cycle is appropriate. To design and challenge a sampling device, we established a reference autoclave cycle for development with the following parameters: 134 °C, one hour, fast exhaust, and deep vacuum (Figure 2). Those very stringent parameters are most commonly used for this type of application. They require compatibility with the vast majority of cycles used across applications.
Regulatory Requirements
These guidelines are applicable for sampling containers to be used in the biopharmaceutical industry.
Extractable Study: USP chapter <661> Non Volatile Residue, Metal Analysis, Buffering Capacity and USP 33/NF28 Containers — Plastics
Bacterial Endotoxin: USP 33/NF 28, chapter <161> Transfusion and Infusion Assemblies and Similar Medical Devices.
Cytotoxicity Test: ISO 10993, Part 5 Biological Evaluation of Medical Devices, Part 5 (2009)
USP 32/NF27, chapter <87> Biological Reactivity Tests, In Vitro.
Biological Reactivity Tests for Class VI Plastics: USP <88> Biological Reactivity Tests, In Vivo and USP 32/NF 27 for Class VI Plastics

Impact on Sampling Device Design
As Figure 2 shows, pretreatment is required during an autoclave cycle to displace as much dead volume of air as possible and replace it with steam. One method to achieve this is to remove the air by creating a rapid vacuum. Doing so typically causes no damage to stainless steel devices. Nevertheless, any device placed in an autoclave for such a cycle must be equipped with an adequate vent to allow a flow of gas without limiting the change of pressure. The vent itself cannot be stressed and must maintain its integrity during the process. Failure to take this into consideration can lead to a catastrophic collapse or bursting (Photo 1).
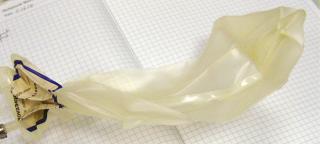
An ideal sterile sampling device would be a presterilized closed container. When such an item needs venting, a question arises regarding the risk of contamination. So the vent must be chosen properly and must maintain integrity throughout the life-cycle of each device. If a sampling device has already been presterilized by irradiation, it obviously does not need resterilization. But because such a device is made of deformable material, any decrease of pressure during an autoclave cycle will cause air inside the container to expand. If venting is impossible, stress will inevitably occur (Photo 2). A rapid decrease of pressure combined with no or little venting will cause a device to inflate, deteriorate at its most vulnerable part, and ultimately rupture (Photo 3). Venting is the only way to make disposable, flexible containers that are compatible with their use for sampling from equipment sterilized by autoclaving (Photo 4).
Photo 3:
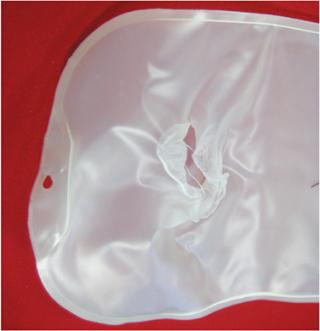
Photo 4:

Dwell sterilization allows for maintenance of a constant temperature (and therefore a constant pressure) over a defined period. A sampling device made of plastic material will more quickly reach the expected sterilization temperature than will a vessel and piping made of stainless steel. Without a check valve or equivalent venting device, the pressure will push the device’s internal faces together. The combination of heat and pressure could cause the inner walls of a flexible bag to adhere irreversibly together, making such a sampling device unusable (Photo 5).
Photo 5:
To prevent such melting, sticking, and damage, the polymer-film properties are as critical as a proper venting mechanism.
For rapid equipment drying, a poststerilization treatment is performed. Rapid variations of pressure will create a violent draft that causes water to be expelled — when a vessel is adequately designed. As a result, such treatment also induces significant stress for flexible containers. Moreover, during poststerilization treatment, steam is replaced by air. If that air is not dry and/or if the temperature decreases too rapidly, condensation will occur. Again, proper venting (using if possible a hyperhydrophobic membrane) will limit or prevent such condensation fr
om appearing in the container.
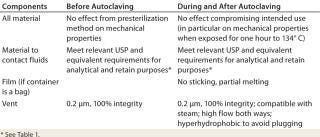
Autoclaving flexible containers drives the requirements listed in Table 1. Few thermoplastic resins available on the market are considered to be compatible with both autoclaving and ionization by radiation. So validation data are not easy to obtain. When they are available, autoclave specifications are frequently for 121 °C (the minimum temperature), and irradiation specifications are typically 25 kGy (a small dose). In practice, many operators use higher temperatures (123–134 °C) and irradiation doses that are >25 kG. Manufacturers of sampling devices need to be aware of this problem.
Table 1: Requirements driven by the use of flexible containers in association with an autoclave
Striking the Best Balance
Delivering the benefits of single-use sampling devices that are fully compatible with autoclaving is a significant challenge. Both designers and users of single-use sampling devices must balance all parameters to achieve an optimum outcome. Those include biocompatibility specifications and autoclave compatibility (high temperatures, long exposure times, multiple vacuum cycles and fast exhaust speeds) as well as a guarantee of sterility. Once film, resins, tubing, and raw-material candidates have been selected, each of them must be evaluated against each of those parameters. An additional challenge is faced in designing properly sized vents that are both compatible with gamma presterilization and autoclaving. The last step is to ensure the manufacturability of a device that meets specifications driven by required performance while ensuring that the demanding requirements of a biopharmaceutical industry customer will be met at an acceptable cost.
The Outcome: Ultimately, we have developed a range of single-use sampling containers that can be autoclaved along with their host stainless-steel containers up to 134°C for one hour while experiencing rapid changes of pressure. These containers are both flexible and rigid, with sizes ranging 5–1,000 mL. Expected improvements in materials should make this method more commonly and extensively available for use in desired applications.
About the Author
Author Details
Michèle Dumon is a senior R&D engineer for Mobius hardware, 33-3-9046-9562, fax 33-3-9046-9141; [email protected]. Nadine Wendenbaum is an R&D validation engineer in the validation and application center; 33-3-9046-9480, fax 33-3-9046-8890, [email protected]. Both are at Merck Millipore SAS, Bâtiment B, 39 Route Industrielle de la Hardt, 67120 Molsheim, France.
1.) Module 1 Basic Principles of GMP: (Part 13) Good Practices in Production and Quality Control 2006. WHO Basic Training Modules on Good Manufacturing Practices (GMP), World Health Organization, Geneva.
2.) GUI-0001 2009. Good Manufacturing Practices (GMP) Guidelines: 2009 Edition, Version 2, Health Canada, Ottowa.
3.) ISO 17665-1:2006 2006.Sterilization of Health Care Products — Moist Heat — Part 1: Requirements for the Development, Validation and Routine Control of a Sterilization Process for Medical Devices, International Organization for Standardization, Geneva.
4.) ISO/TS 17665-2:2009 2009.ISO/TS 17665-2:2009. Sterilization of Health Care Products — Moist Heat — Part 2: Guidance on the Application of ISO 17665-1, International Organization for Standardization, Geneva.
You May Also Like