Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
June 1, 2014
https://bioprocessintl.com/wp-content/uploads/2014/06/062014-Masser-abstract.mp3Downstream protein purification (the stage in which a protein is isolated and purified) is one of the last steps in biotherapeutic manufacturing. Single-use technologies are an increasingly popular choice for both upstream and downstream bioprocessing because they offer significant benefits over traditional multiuse manufacturing systems. Single-use technologies also provide an array of logistical benefits, including reduced costs, minimized risk of cross-contamination, and improved operational efficiency (1).
Challenges remain, however, in designing a complete, streamlined, single-use process for downstream protein purification. In-process sensor technologies, chromatography, and temperature control are all areas that have been traditionally underserved by single-use technologies and represent “weak points” in the downstream process flow. Exacerbating the problem, most proteins are heat labile and sensitive to a large number of environmental conditions. Therefore, interruptions in the process and improper temperature management can increase aggregation (which can lead to unstable formulations) or even degradation and denaturation in worst cases.
Product Focus: Proteins
Product Focus: Purification
Who Should Read: Downstream process development, engineering
Keywords: Filtration, chromatography, heat transfer, single-use sensors
Level: Intermediate
Meanwhile, significant strides have been made in upstream process development. Large-scale bioreactors have been introduced, thereby increasing the volumes of initial cell cultures. New cell lines have been developed to increase titers. In addition, a number of bioreactors have been introduced that address some common bioprocess challenges (1,2). Improvements in culture media and supplements, connection technologies, disposable mixing systems, integrity tests, and sampling processes all have had a positive effect on the capacity of upstream bioprocesses, as well as the expression level of some proteins.
Such upstream improvements have “outpaced” advances in downstream technologies, thereby starting a bottleneck effect. Such cases have been especially prevalent in facilities running end-to-end biomanufacturing. The potential gains in capacity brought on by upstream innovations cannot be appreciated fully as long as downstream technologies play “catch up.” That disparity is reflected in the concerns of biomanufacturers, with 57.5% of respondents to a recent survey reporting that better downstream technologies are needed to prevent capacity constraints (3).
Here we discuss several downstream processes in which new technical solutions are in high demand, particularly sensors, chromatography, and heat transfer. We also
review how suppliers across the bioindustry are filling such single-use technology gaps, improving the speed and efficacy of protein recovery, and helping to alleviate downstream bottlenecks.
Many of the first single-use sensors were geared toward upstream processes, measuring parameters such as pH, dissolved oxygen, and glucose content during cell culture. However, sensors aimed at downstream processing are now starting to become commercially available.
Several critical process parameters should be monitored during downstream processing, including pressure, temperature, flow-rate, conductivity, pH, turbidity, and UV absorbance. Accurate sensor technologies that are compatible with single-use processes would facilitate alignment with the FDA’s process analytical technologies (PAT) initiative (4). The agency introduced PAT to promote in-line monitoring and control of manufacturing processes and encourage manufacturers to make corrections in the course of their processes, in accordance with the FDA’s overall quality by design (QbD) initiative.
In response to PAT, several companies have developed in-line real-time sensors. In most instances, sensors must be insensitive to a number of chemicals and solvents, and they must be robust to withstand a range of process conditions. Because of the increasing popularity of single-use technologies, sensor suppliers offer products in noninvasive or single-use formats. They also face challenges with expense: If a sensor is too expensive, then the cost benefits of single-use technology may be eliminated.
Temperature is one of the most critical process parameters in bioprocessing, so a wide array of single-use temperature sensors have been engineered. Such sensors (measured most commonly with an RTD, thermocouple, or thermistor) have advanced such that several options are now commercially available.
Flow can be calculated indirectly (by measuring a related property and correlating it to flow rate). Because proteins absorb UV light at 280 nm, suppliers have developed flow-through UV sensors. Studies with such technologies have shown favorable results comparable with measurements taken using traditional stainless steel flow cells (5).
Chromatography presents particular challenges for disposable technologies. Numerous points of contact with valves, pumps, and monitors are present in standard chromatography systems. The generally high cost of chromatography matrices is another drawback. Some “disposable” columns are in practice; however, in some cases, disposable columns are used repeatedly to lessen the frequecy of purchasing new columns.Such practice has potentially deleterious effects. Proteins can be especially demanding for chromatography, because their size restricts free movement into the micropores of resin beads in traditional columns.
Cation-exchange chromatography (CEX) is becoming an increasingly popular option (6) because it offers several advantages over traditional methods at a significantly lower price point. An CEX chromatographic matrix is an order of magnitude less expensive than a typical protein A affinity matrix. Moreover, the dynamic binding capability of CEX is significantly higher than that of protein A, and CEX does not require a low pH during elution.
Concern about protein size helped prompt the development and use of membrane adsorber chromatography. Charged ligands bind target particles, and as a result there is no need for diffusive migration into resin beads. An open pore structure (5–8 µm) offers little resistance to nontarget particles. Higher flow rates shorten processing times and decrease in hold-up volumes in single-use chromatography devices.
High-performance tangential-flow filtration (HPTFF) enables purification, concentration, and buffer exchange to be performed in a one-unit operation (7). It is a two-dimensional purification method that exploits differences in both the size and charge of proteins and other biomolecules. As a result, it can even separate two proteins that have the same molecular weight.
Another alternative is the use of simulated moving bed (SMB) technology. Here, the solid phase is placed in columns that are connected in series, with the mobile phase running countercurrent. Inlet and outlet valves move continuously to replicate a moving bed. SMB was developed 50 years ago but has recently found application in single-use bioprocessing. It uses less resin and solvent than other technologies, but it is complex technology and requires high up-front costs.
Temperature Control and Heat Transfer: Temperature is one of the most important factors in downstream processing. Specifically, manufacturers must have the ability to precisely control the temperature of a process solution as it enters or exits a processing step (such as chromatography). Finite temperature control of process solutions entering chromatography directly correlates with the amount of protein recovered. The ability to rapidly cool (or heat) a process solution in real time as it passes through a heat transfer device (rather than collecting process solution for off-line cooling) has an obviously positive effect on overall processing time.
Temperature control and effective heat transfer can improve processes and help alleviate downstream bottlenecks.
Common points at which this is particularly true include clarification, chromatography, and ultrafiltration/diafiltration.
Harvest clarification involves processing large batch volumes and is typically performed using continuous-flow centrifugation or depth-filtration. It might require an extended processing time, which can result in increased temperature. Typically, manufacturers use a jacketed mixer or hold station between clarification steps and column loading to control temperature.
Chromatography is a crucial factor in the final yield of a protein product. Although 100% recovery is never possible, proper optimization of chromatographic separation will often dramatically improve recovery rates, especially given that purification requires multiple rounds of chromatography in many instances. Loading conditions critically affect protein recovery. It is necessary that process solution and loading buffer be at the same temperature. Without proper temperature control, nonspecific binding and reduced recovery will result.
Polishing from ultrafiltration/diafiltration (UF/DF) is another important determinant of protein yield. Proper temperature control of process solutions can substantially improve recovery. In the absence of a single-use solution, process engineers are obligated to choose between one of two suboptimal options. The first is to insert a stainless steel multiuse exchanger (e.g., a tube in shell system) into the process. That method does not take advantage of many of the benefits of single-use systems, because part of the equipment requires a lengthy clean-in-place or steam-in-place (CIP/SIP) cycle, which can extend downtime and exacerbate downstream bottlenecks. The other option is to use a jacketed mixing tote. Jacketed mixing totes are not designed for enacting rapid temperature changes of solutions. Because of the low heat-transfer rate, the process requires a significantly greater period of time, thus extending process time and exacerbating downstream bottlenecks.
In 2013, ASI introduced the bioindustry’s first single-use heat exchanger. It is a long-awaited technological improvement that offers significant benefits to both upstream and downstream processing, and significantly helps to alleviate protein purification bottlenecks.
The DHX heat exchanger (Photos 1 and 2) is a modular system based on widely accepted industry standard plate and frame designs that use bags to create a sterile fluid path. Such bags fit tightly between five stainless steel plates. The process fluid flows through single-use bags, whereas the heating or cooling fluid runs in a countercurrent through the plates in a completely isolated fluid path. Dimpled jacketing on the plates helps to create a turbulent flow and maximizes heat transfer. Table 1 lists some key technical specifications for the DHX heat exchanger.

Photo 1: DHX single-use heat exchanger shown with plates open and plates closed

Photo 2: DHX heat exchanger; single-use fluid path, with high heat-transfer ratesIs

Table 1: Technical specifications of the heat exchanger
In addition to protein purification, the DHX system is applicable for harvest hold, buffers, and bulk drug substance. It also is suitable for upstream applications, including media hold, fermentation, cell separation, protein harvest, harvest cooling, and harvest hold.
Figure 1 and 2 show the cooling and heating efficiencies, respectively, as a function of flow rate (liters per minute, LPM). Figure 3 and 4 display cooling and heating rates, respectively, for different LPM values. In all cases, heat transfer rates for both heating and cooling are comparable
with existing stainless-steel heat exchangers.
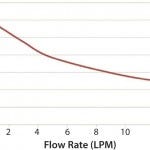
Figure 1: Cooling from 37 °C, single pass; process fluid is water at 37 °C; heat-transfer fluid is 30% propylene glycol at 2 °C; number of bags = 4; flow rate = 15 LPM.
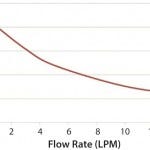
Figure 2: Heating from ambient temperature, single pass; process fluid is water at 20 °C; heat-transfer fluid is 30% propylene glycol at 42 °C; number of bags = 4; flow rate = 15 LPM.
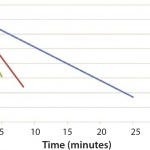
Figure 3: DHX cooling efficiencies measured by temperature and time
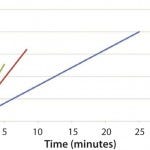
Figure 4: DHX heating efficiencies measured by temperature and time
By accurately controlling the temperature of a process solution before and after filtration and chromatography steps without stainless steel equipment, the following benefits can be obtained.
Reduced Infrastructure Needs: A heat exchanger that integrates into a single-use process obviates the need for a cold or warm storage room. This decreases the complexity of a manufacturing facility’s requirements and concomitant costs.
Reduced Bioburden: A sterile, temperature-controlled fluid path for process solutions, buffers, and other process components addresses weak points in a CIP or sanitation process that are typically required for stainless steel equipment.
Rapid Turnaround of Process Equipment: Time-consuming CIP (and often SIP) operations are not required when using single-use heat exchangers. That eliminates cleaning and sterilization steps that can decrease turnaround time from days to hours and allows expensive capital equipment to be reused.
Taken together, recent developments in sensors, chromatography, and heat transfer technologies have gone a long way to address the downstream bottleneck and improve efficiencies in end-to-end bioprocessing. These new developments when properly implemented by process engineers can be expected to increase yields, improve quality, reduce infrastructure requirements, lower contamination risks, and decrease costs in single-use bioprocessing.
Author Details
Derek Masser is director of sales, life sciences at ASI, 163 Research Lane, Millersburg, PA 17061; 1- 717-692-2104 ext. 256; [email protected].
1.) Shukla, A, and Gottschalk, U. 2013. Single-Use Disposable Technologies for Biopharmaceutical Manufacturing. Trends Biotechnol. 31(1):147-154
2.) Allison, N, and Richards, J. Dec 24, 2013. Current Status and Future Trends for Disposable Technology in the Biopharmaceutical Industry. J. Chem. Technol. Biotechnol http://onlinelibrary.wiley.com/doi/10.1002/jctb.4277/full
3.) Langer, E. 2012. A Decade of Biomanufacturing. BioProcess Int. 10(S6):65-68
4.) Guidance for Industry. 2004. PAT — A Framework for Innovative Pharmaceutical Development, Manufacturing and Quality Assurance. Fed. Register. 69(191):59257-59258.
5.) Renault, P, and Annarelli, D. 2013. Evaluation of a New Single-Use UV Sensor for Protein A Capture. BioProcess Int. 11(2):48-51.
6.) Marichal-Gallardo, PA, and Alvarez, MM. 2012. State-of-the-Art in Downstream Processing of Monoclonal Antibodies: Process Trends in Design and Validation. Biotechnol. Prog. 28(3): 899-916
7.) van Reis, R. 1997. High Performance Tangential Flow Filtration. Biotechnol. Bioeng. 56(1):71-82
You May Also Like