Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
June 1, 2009
What’s keeping senior biopharmaceutical executives awake late at night? According to BioPlan Associates, Inc., which publishes an annual comprehensive survey of the state of worldwide biopharmaceutical manufacturing, capacity constraints are among the key issues at hand (1). And one of the most important constraints is the lack of physical capacity in purification equipment. Bioreactors are producing a lot more protein than current downstream purification steps are designed for. Overcoming the resulting bottlenecks may require increasing the productivity of downstream unit operations and turning over processing equipment faster to handle each consecutive batch.
Many biopharmaceutical manufacturers are turning to single-use systems to increase product throughput. Recent advances extend the use of disposables from the typical bioreactors and buffer or media storage bags to clarification and chromatography steps – and even to viral filtration and drug formulation. With such advances, the industry is on the road to realizing a fully disposable purification process, at least for the 2- to 3-kg scale.
Costly Cleaning Steps
Cleaning and cleaning validation are some of the most time-consuming and least revenue-generating activities in biopharmaceutical manufacturing. The costs associated with cleaning and waste treatment are significant. A recent article reported that single-use facilities can consume up to 87% less water than traditional facilities, which represents significant savings (2).

Figure 1:
ILLUSTRATION BY CHERYL SCOTT USING PHOTOS FROM STOCKXCHANGE (WWW.SXC.HU)
Cross-contamination is another issue worrying drug manufacturers, particularly in multiproduct facilities. The US Food and Drug Administration (FDA) recently released a guidance document for phase 1 investigational drugs, which mentions this concern (3). Noting the susceptibility of a phase 1 investigational drug to contamination or cross-contamination with other substances, the FDA lists technologies and resources that can facilitate conformance with CGMPs and streamline product development. Examples cited include using disposable equipment and process aids to reduce cleaning burdens and contamination risks and the use of commercial, prepackaged materials either to eliminate the need for additional equipment or to demonstrate demonstrating CGMP control of existing equipment.
Another factor affecting the trend toward disposable systems is tight financing. For manufacturers that can’t find the funds to buy traditional processing equipment, one alternative is to develop facilities that require less overall expense. Small companies with tight cash flows, companies that use contract manufacturers, or companies that set up their own single-use facilities may find a single-use system far cheaper to pull together, less capital intensive, and faster to set up than a traditional facility.
Steps Along the Road
Figure 1 shows the generalized steps in a bioprocess at 2- to 3-kg scale, indicating a number of steps in which single-use systems are fully developed and currently being used by manufacturers. These include bioreactor and buffer storage, for which single-use systems are being used extensively, as well as many other areas in which single-use unit operations are less common but available. The latter include clarification, chromatography, virus clearance, and final formulation. In the purification steps not highlighted, single-use technologies are advancing but not yet fully developed.
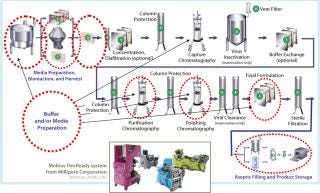
Figure 1: ()
Media Filtration and Buffers: According to the BioPlan report, average spending on single-use products increased for all categories, with the largest percentage increases in mixing systems (237%), media bags-dry (66%), and bioreactors (43%)(1). Single-use solutions for buffer and media preparation maximize equipment use and optimize capital expenditure, particularly in multiproduct and multiscale facilities. Disposable mixers for mixing buffers and for hydration of cell culture media are available from ThermoFisher (HyClone), Millipore, and ATMI/LevTec.
Millipore’s Mobius FlexReady solution (pictured above) for buffer or media preparation features disposable mixers of 100 L to 500 L in scale. The solution incorporates presized filters for typical cell culture media and buffer filtration applications for a range of volumes. This fully integrated system can be quickly changed to accommodate different process needs including varying volumes. It also features disposable, appropriately-sized product collection bags and filter flush bags.
HyClone offers a single-use mixer (SUM) system based on the same principles as its single-use bioreactor (SUB) system. The SUM version allows mixing in a closed system by using a sterile SUM bioprocess container and is appropriate for 200-L, 500-L, and 1,000-L applications. The Newmix-LevTech product line from ATMI offers a broad range of scalable, flexible, single-use mixing systems in sizes from benchtop to industrial scale. They are based on an electromagnetically levitated impeller that allows a fully closed system.
Bioreactors: Single-use bioreactors up to the 1,000-L scale containing full instrumentation with pH, dissolved oxygen, and temperature sensors are used as seed reactors and to some extent as production bioreactors, particularly for high-titer expression systems and high-density cultures. For example, in GE Healthcare’s Wave Bioreactor cells and media are contained in a fully disposable CellBag that can hold volumes up to 500 L. HyClone/ Thermo Fisher Scientific, Sartorius-Stedim Biotech, and Xcellerex offer a range of choices for single-use bioreactors.
Clarification: In the clarification step, single-use systems can adapt to changing surface areas and media grade requirements as multiple products come through the development pipeline. In addition, such systems eliminate the need for significant capital investment in a centrifuge.
For example, Millipore’s solution for clarification uses Millistak+ depth filtration media for cell-culture clarification directly from a bioreactor. The system is designed to accommodate up to 5.5 m2 of depth filter and sterilizing filtration media downstream. Depending on cell density and viability, this system can be used to clarify 10-L to >500-L bioreactors.
Purification and Polishing Chromatography: Single-use chromatography columns are prepacked, presanitized, and prequalified. No testing is required before a run, and no cleaning or validation is necessary. A wide range of media are supplied prequalified with packing and testing data. For example, the simple exchange of the complete flow path of GE Healthcare’s ÄKTA ReadyToProcess system eliminates a need for system cleaning (including method development and validation).
For years, membrane adsorbers have been on the market to replace flow-through chromatography columns for polishing chromatography. Pall’s Mustang-Q, Sartorius-Stedim’s Sartobind-Q, and Millipore’s ChromaSorb devices eliminate completely the need for packing and cleaning of chromatography media in large columns.
Viral Filtration and Clearance: Fully integrated single-use systems for virus clearance are a relatively new addition to the disposables arena. The Millipore solution for virus clearance features Viresolve Pro parvovirus removal filters for rapid and efficient parvovirus clearance from protein solutions. By using a pump-based filtration system (rather than a pressure-vessel based one) for constant-pressure operation, this solution offers maximum flexibility for use in multiscale and multiproduct facilities. The system can handle protein loads of 10–1,000 g depending on the filter area used and validated protein loading.
Another virus-filtration example is the Sartorius Virosart CPV filter, with protein transmission >95%, >4 log10 for PPV and >6 log10 for retroviruses. This system is scalable from 5.3 cm2 up to full-scale production sizes. Such systems have been embraced by the industry because of their assurances of high virus retention and fast, efficient processing.
Drug Formulation: The Millipore solution for tangential flow filtration (TFF) offers a fully disposable flow path that includes retentate vessels with active mixing and high-performance Pellicon 3 filters. The system features a novel single-use retentate bag with low-point retentate return, a vortex breaker, and a levitating magnetic impeller – all features that are typically found in stainless steel vessels. A low–dead-volume T connector enables use of traditional pressure transducers and eliminates the need to clean disposable pressure transducers in place. The hardware and disposable flexware in this system are engineered to provide high protein recovery at high concentrations. Such a system offers improved economy and productivities because of simple procedures and low down-time between products or batches.
Pall’s Kleenpak TFF MF capsule combines disposable capsule technology with TFF for microfiltration applications such as cell clarification. This autoclavable device includes a dual-layered, low-adsorption Supor membrane with an area of 0.50 m2 (5.4 ft2) and a high flux rate for maximized product recovery. Capsules can be manifolded in series and in parallel to increase the effective filtration area of an overall system. They are engineered to meet the biopharmaceutical industry’s requirements – including low extractables – and are validated by the manufacturer.

Single-Use System Challenges
Adopting single-use solutions for all bioprocess steps will not happen without some significant challenges. First on the list is a limitation of scale. At present, it is impractical to use disposables at a very large scales, primarily because of the limited availability of a robust and cost effective pumping solution at flow rates >20 L/min.
The second issue here is the overall robustness of single-use systems. The biopharmaceutical industry has years of experience perfecting its use of traditional stainless steel processing equipment. Given the more recent introduction of disposable processing equipment, it will probably take some time to make disposable systems comparably robust, but it is imperative that manufacturers do so. A buffer that leaks is a nuisance; a product leak can be a show stopper. Vendors of single-use products need to make sure that their systems are well designed, qualified, and tested.
Cost is often discussed as a challenge. Increased adoption of disposable products will drive their costs down somewhat, but some people think single-use processes are and will remain more costly than their reusable counterparts. Although expendable costs are higher with disposables, evidence suggests that the overall ownership cost of single-use systems may actually be comparable to ��– if not lower than – traditional systems (2).
Sustainability has been mentioned as another obstacle to implementation of disposables in bioprocessing. However, single-use systems do not carry the larger carbon footprint that has been attributed to them when total waste (rather than just visible waste) is taken into account. With that considered in full, some single-use systems could even have a net positive impact on a facility’s carbon footprint when compared with traditional stainless steel facilities.
Other challenges include the identification, management, and control of leachables and extractables; the need to improve systems engineering of both disposable and nondisposable parts in single-use purification systems; and the need for more reliable pH, flow, and ultraviolet (UV) sensors for use within such systems.
Developments in Upstream Processing: One factor that may tip the balance toward disposables is advancement in upstream processing. Recent developments �– such as cell-line improvement – have led to high product titers, which have increased pressure on downstream operations. In addition to higher protein mass to process, the potential for aggregates increases at higher expression levels. In some cases, aggregate clearance may require the use of a hydrophobic-interaction chromatography (HIC) column. Depending on the nature of those aggregates, they could be cleared with either flow-through HIC columns or membrane adsorbers. In either case, single-use systems can be designed to efficiently handle the problem.
Given all the recent advances in single-use technology, it is not unreasonable to believe that biopharmaceutical companies will be using fully disposable purification processes in the near future – at least for 2- to 3-kg scale operations – and eventually perhaps at larger scales. Time and cost savings can be substantial for those who implement these solutions, and a number of well-regarded vendor companies exist to work with their customers to perfect the technology.
1.) 2008.Fifth Annual Report and Survey of Biopharmaceutical Manufacturing Capacity, BioPlan Associates, Rockville.
2.) Sinclair, A. November 2008. The Environmental Impact of Disposable Technologies. BioPharm Int. 21.
3.) U.S. Food and Drug Administration July 2008. Guidance for Industry: Current Good Manufacturing Practices (CGMPs) for Phase I Investigations Drugs, Rockville.
4.) Langer, E 2009. Trends in Single-Use Bioproduction: What Users Are Saying. BioProcess Int. 7:S6-S8.
5.) Martin, J. 2009. Organizing the Organizations of Single-Use Manufacturing. BioProcess Int. 7:S9-S16.
6.) Mauter, M 2009. Environmental Life-Cycle Assessment of Disposable Bioreactors. BioProcess Int. 7:S18-S29.
7.) Mardirosian, D. 2009. Scaling Up a CHO-Produced Hormone–Protein Fusion Product From Robotic Optimization to Large-Scale, Single-Use Stirred-Tank Bioreactors. BioProcess Int. 7:S30-S35.
8.) De Wilde, D. 2009. Bridging the Gap from Reusable to Single-Use Manufacturing with Stirred, Single-Use Bioreactors: A Development Approach Based on the Gold Standard. BioProcess Int. 7:S36-S41.
9.) Chapman, P 2009. Integration Is the Future of Single-Use Technology. BioProcess Int. 7:S42.
10.) Rawlings, B, and H Pora. 2009. A Prescriptive Approach to Management of Solid Waste from Single-Use Systems. BioProcess Int. 7:40-47.
11.) Albert, K 2009. Automated Liquid Handlers As Sources of Error. BioProcess Int. 7:48-52.
12.) Scott, C 2009. Pursuing Excellence: Manufacturing Biologics and Drugs. BioProcess Int. 7:S46-S52.
13.) Kuhlman, P. 2009. Rapid Purification of Lys-C from Lysobacter enzymogenes Cultures: A Sequential Chromatography Technique. BioProcess Int. 7:28-38.
14.) Monge, M 2009. Developing Best Practices for Disposables: Current Industry Status. BioProcess Int. 7:64.
15.) Rawlings, B, and H Pora. 2009. Environmental Impact of Single-Use and Reusable Bioprocess Systems. BioProcess Int. 7:18-26.
16.) Pora, H, and B Rawlings. 2009. Managing Solid Waste from Single-Use Systems in Biopharmaceutical Manufacturing. BioProcess Int. 7:18-25.
17.) Decaria, P, A Smith, and W Whitford. 2009. Many Considerations in Selecting Bioproduction Culture Media. BioProcess Int. 7:44-51.
18.) Botterill, M, and B Rawlings. 2008. Applying Good Engineering Practices to the Design of Single-Use Systems. BioProcess Int. 6:18-25.
You May Also Like