Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
With combined 2009 revenues estimated to be over US$40 billion, monoclonal antibody (MAb) products have become the dominant component of the biopharmaceutical market (1). Continued interest and development of this exciting class of products will drive the overall sales of biopharmaceutical products in the future, making them the fastest growing segment of the overall pharmaceutical market (2). To help companies developing MAb products, BioProcess Technology Consultants recently published a comprehensive report outlining the complex technical, regulatory, and strategic chemistry, manufacturing, and control (CMC) activities necessary to successfully advance new MAbs from discovery to first-in-human clinical trials and the market as quickly and economically as possible (3). As discussed in the report, numerous interconnected tasks must be completed cost effectively and on schedule to enable human clinical testing of a new MAb candidate. Those tasks include construction and testing of production cell lines, development of a suitable manufacturing process and suitable analytical methods to produce and characterize the antibody and ensure safety and desired functionality, and production of good manufacturing practice (GMP) material for use in human clinical trials.
Figure 1 shows a typical timeline for CMC development activities required before filing an investigational new drug (IND) application (3,4). This timeline is based on the assumption that development is carried out in the context of a reasonably well-established platform process for MAb product manufacture and a set of analytical methods for product characterization and quality control (QC) testing. Because these initial CMC activities are on the critical path to an IND filing, it is essential that they be completed as quickly as possible to facilitate the initiation of human clinical trials.

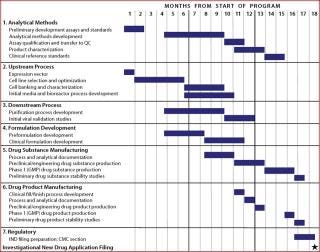
Figure 1: ()
Each major group of tasks in Figure 1 is generally performed by distinct functional groups, either within a product development company or outsourced to service providers. All technology developed before the first CGMP manufacturing run — such as the production cell line, analytical methods, and cell culture and purification processes — must be transferred to a manufacturing and product testing site(s). Later in product development, manufacturing processes and analytical methods are often transferred from an initial manufacturing site to facilities that can produce larger batches of product. Whether such transfers occur within a company/facility or between organizations (such as from a company to a service provider), careful planning and management are essential to ensure the knowledge and experience gained during development and early and clinical manufacturing is effectively transferred. That enables the receiving organization or party to quickly and effectively perform the critical elements of transferred methods or processes (5).
Tech Transfer Considerations
For efficient and successful technology transfer, planning should begin at a very early stage and be driven by the target product profile (TPP). A TPP describes a product’s intended use and quality targets to ensure that product development begins with the end product in mind and that a final product meeting the objectives of the TPP is delivered to the clinic — and eventually to the market. The purpose of the TPP is “to provide a format for discussions between a sponsor and the FDA” regarding potential label claims and characteristics of a new product. The TPP is a dynamic summary of product characteristics that is subject to revision as a product moves through development (6). Nevertheless, it will help define the critical product characteristics that must be maintained or met during technology transfer, allowing the transfer plan to be clearly delineated and thereby preventing ambiguity between the sending and receiving parties.
A technology transfer plan should outline all transfer activities in detail, including the timing of initiation for each transfer activity, the interdependence of various activities, and the criteria for determining successful technology transfer. Each activity should be defined in sufficient detail so receiving parties can plan accordingly and allocate appropriate resources to successfully complete it within a specified timeframe. For most technology transfer programs — such as transfer of a manufacturing process — a project kick-off meeting should be held before the technology transfer plan is finalized to delineate transfer activities, timelines, and responsible parties for all sub-activities. Both the sending and receiving parties must agree on the plan details.
A technology transfer plan also should outline an appropriate governance structure to oversee all transfer activities and ensure that information is shared appropriately over the course of the transfer process. With a cross-company governance team established early in the project, challenges can be readily addressed or prevented to facilitate the process. This governance team also ensures proper buy-in from both parties for the transfer activities and helps with staff allocation, overseeing the transfer project budget and coordination of related activities.
In addition to the technical details of a process being transferred, the regulatory expectations and responsibilities of both parties also should be clearly defined. This includes identifying what regulatory submissions will be required as a result of the transfer and who will be responsible for preparation of those documents. Regulatory expectations and responsibilities can be outlined in the technology transfer plan, but they also should be concisely captured in a formal quality agreement (also known as a technical agreement) between the two companies in an outsourcing arrangement.
The quality agreement is a legal document that defines the relationship of two or more parties with respect to their quality responsibilities, with the business objective being a service or product supply. In the European Union, such agreements are not only an expectation, but also a regulatory requirement (7,8). In the United States, quality agreements are not required by the FDA, but the agency does expect sponsors and contractors to have a formal quality agreement in place, and inspectors often want to review such agreements during regulatory inspections. The fact that FDA has cited sponsors for violations in their quality agreements with contractors demonstrates the importance of having such agreements and ensuring that they accurately reflect each party’s roles and responsibilities (9).
Once the initial prospective planning is complete, the technical portion of a technology transfer begins with assembling a summary of all prior knowledge about the cell line, method, product, or process being transferred. The team identifies critical quality attributes (CQAs) and critical process parameters (CPPs) that define the process and need to be controlled during each production batch.
As the foundation of product knowledge and understanding, CQAs are the critical molecular and biological characteristics for ensuring safety and efficacy of a product. When transferring an early stage product, the CQAs are unlikely to be firmly established yet because both product and process knowledge are minimal. For a broad class of products such as MAbs, however, defining what the product attributes should be can be based on general properties of antibodies and on established safety parameters for all biopharmaceutical products.
Likewise, in early stages of process development, the CPPs most likely to affect the quality attributes of a product are unlikely to be well defined. This can make early stage transfers difficult because no robust data set can be referred to for troubleshooting. However, at this point, the TPP serves as a guide to capture product attributes (e.g., purity and stability) as they emerge. To establish the process under these circumstances, additional work may be necessary to verify that a process has been transferred correctly and to collect the knowledge through design of experiments (DoE) at the receiving site.
If the process being transferred is relatively well understood, then process verification (simply running the process as defined) may be sufficient to establish it at the receiving site. To facilitate process verification, prepare a detailed description of the current process that clearly describes each of the major operational steps being transferred. This process description should generally follow the format of a high-level batch record, but without the details of a complete batch record. Alternatively, development reports that describe the process can be used in lieu of preparing a separate document. In either case, the process description will allow a technology transfer team to review preliminary documents and confirm that all necessary information for each unit operation or process step has been accurately and completely documented.
The existing process is then transferred into a development or pilot laboratory and run as closely as possible to verify that the receiving site can reproduce the process. Results from the process verification run(s) determine how much — if any — process adaptation is required to fit the process to a receiving facility. For early stage transfers, adapting the process to the facility may be more appropriate because a large body of process data will not yet exist. Such process adaptation might involve reducing the elution volume from a chromatography step to allow an existing vessel to hold it. However, for later-stage or commercial transfers, adapting the facility to the process (within reason) is more appropriate because the risk of affecting a CQA/CPP is greater if a process is changed at that point.
Analytical Methods
Given the complexity of MAb products, a broad range of analytical methods is essential for full characterization and QC testing during GMP manufacturing. Successful transfer of these analytical methods is a critical component of technology transfer — whether an entire process is being transferred or just the analytical package. In an entire process transfer, analytical methods will be transferred as part of the overall program.
However the process and analytical method transfer teams are often made up of different people. If their activities are not well coordinated, then product manufacturing could be delayed — if, for example, analytical method transfer has not kept pace with process transfer. To minimize the potential for such delays and to keep both teams in sync, a dedicated assay transfer list should be developed in parallel with the process transfer list. Analytical methods should be divided into those required for product release and those used for product characterization, with priority given to the transfer of the product release assays.
Table 1 lists typical product release assays for a MAb product. The exact analytical methods used for the testing and release of a MAb drug substance and drug product (and the specifications for each method) are determined based on process development history, product characteristics, and regulatory requirements. Because multiple analytical methods may be available for assessing different product attributes, not all methods are used for every product. Although methods measuring product attributes that may affect the safety or efficacy of a product are required, developers have flexibility in determining which particular analytical methods will be used to determine product identity, purity, or potency.
Table 1: typical product release assays for MAb products
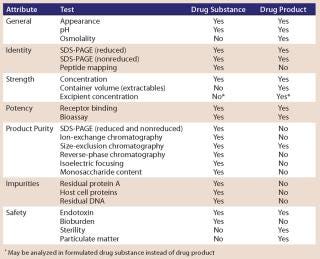
Table 1: typical product release assays for MAb products ()
An analytical method transfer flow chart is a valuable tool for managing this complex transfer. Such charts provide a description of the methods being transferred, timeline expectations, required test performance, result trending, and other critical points to consider as part of the method transfer. In addition, at any stage of method transfer a thorough evaluation of equipment in the receiving laboratory should highlight major differences in available equipment — including the vendor type, which can have an impact on the method transfer results.
As with analytical equipment, manufacturing equipment at the receiving location should be thoroughly evaluated for comparability. Before proceeding with engineering batches, the technology transfer team will determine whether dedicated equipment is necessary or whether existing equipment is inappropriate for the intended process. Also referred to as pilot or demonstration batches, engineering batches are normally not performed in full GMP compliance. This allows for extra in-process sampling, refinement of manufacturing batch records and related GMP documentation, adjustments to the process as required by facility and equipment differences, and a reduction in the cost of the initial, high-risk full-scale production. By running at least one such batch at scale and adjusting some parameters based on the data from that run, a company can reduce the risk of its initial GMP batches failing. Thus, it is more likely that subsequent production runs at the same scale will be reliable, reproducible, and robust.
Ensuring Comparability
If the process or methods being transferred were previously used to make product for human clinical trials, it is essential to establish comparability of material made by the transferred process used in the clinical trials (“postchange product”) to material previously used in the clinical trials (“prechange product”). To determine the potential impact of a technology transfer, the product sponsor should conduct a risk assessment (as outlined in ICH Q9) to evaluate how a process change (or series of changes) on product quality or safety affects the quality or safety of the product. The impact of a technology transfer and associated process change(s) may be difficult to evaluate early in product development and may even be ambiguous later in the product life cycle. However, the later in product development a technology transfer and associated process change(s) takes place, the greater the risk that the postchange product will not be comparable to the prechange product. Table 2 summarizes potential risks associated with a number of common process changes that often occur during a technology transfer of manufacturing and associated CMC activities during early stage development of a MAb product.
Table 2: Potential impact of technology transfer and process change on product comparability during early stage product development

Table 2: Potential impact of technology transfer and process change on product comparability during early stage product development ()
Generally, if the changes required for technology transfer are no more than adapting a process to the receiving facility and equipment, then the risk associated with that technology transfer is low. However, if the technology transfer includes process development, optimization, or scale-up that could affect product characteristics, then the risk associated with that technology transfer may be greater and should be carefully evaluated. Because comparability assessment can be expensive, some companies choose to implement multiple process changes simultaneously during transfer so they can be assessed by a single comparability study. If a product sponsor takes advantage of such an opportunity to implement process and/or method improvements during transfer, then the risk to comparability increases significantly.
Once a comparability study has been executed and its resulting data have been analyzed, the results are summarized in a comparability report. All verification or development data generated during a process transfer is included in such a report, which forms the basis for any regulatory submission that may be required supporting the use of postchange product in ongoing human clinical trials. If study results indicate that posttransfer product is not comparable to that before transfer, then a company can either abandon its transfer — which is unlikely — or perform additional development and/or product characterization work to ensure that the transferred process can make product that is comparable to what was manufactured before the transfer. In a worst case, the company may perform human clinical safety, pharmacokinetic, and efficacy studies using product from the new process.
A Global Perspective: Often, technology transfer will include moving manufacturing to a new site in another country (e.g., a new facility or a CMO overseas). In these cases, the transfer activities and risks associated with them may be even more complicated due to language and cultural differences. Also, the difficulties of managing technology transfer and ongoing manufacturing may be further magnified by time-zone separation. Nevertheless, such transfers are increasingly common practice as biopharmaceutical supply chains become more global in nature. As a result, it is extremely important to establish good communication between the sending and receiving parties. Under the best of circumstances, miscommunications are common — and they can be compounded when sending and receiving parties do not share a common language. In transferring manufacturing to a new territory, it is also important to ensure that compliance with local regulatory requirements in the other country is achieved as well as maintaining compliance with the regulatory requirements of your home location.
Be Prepared
Technology transfers are a necessary component of MAb product development. As such a product succeeds and advances through development, its manufacturing requirements will change. The probability of a smooth and successful technology transfer process depends on several factors. Both sending and receiving parties must be well aligned in their expectations and plans, which can be facilitated by early and detailed planning and communications.
The better a process and product are understood in terms of CQAs and CPPs, the greater its likelihood of successful transfer and demonstration of comparability. Risks should be identified and controlled as best as possible. Transferring a process is an expensive and time-consuming activity, including both the manufacturing and potential nonclinical and clinical activities. Even if both parties follow best practices, there is no guarantee that their transfer will go smoothly and result in a comparable product. Sometimes the best plan is to have a contingency plan as well.
About the Author
Author Details
Corresponding author Patricia Seymour is a senior consultant; Susan Dana Jones, PhD, is vice president and senior consultant; and Howard L. Levine, PhD, is founder, president, and principal consultant of BioProcess Technology Consultants, Inc., 289 Great Road, Suite 303, Acton, MA 01720; [email protected]; www.bioprocessconsultants.com.
1.) 2010. Biomanufacturing Supply and Demand Databases, Bioprocess Technology Consultants, Inc., Acton.
2.) Ransohoff, TC, and HL. Levine. 2009. Capacity Counts. Inside Outsourcing. Pharmaceut. Exec. www.nxtbook.com/nxtbooks/advanstar/insideoutsourcing_200911/index.php?startid=18 29:S18-S23.
3.) Levine, HL and G 2010.The Development of Therapeutic Monoclonal Antibody Products, BioProcess Technology Consultants, Inc., Boston.
4.) Jones, SD. 2010. CMC Activities for Development of Monoclonal Antibody Products. Contract Pharma 12:60-63.
5.) Technology Transfer Task Team 2003.Good Practice Guide: Technology Transfer, International Society of Professional Engineers, Tampa.
6.) CDER 2007. Draft Guidance: Target Product Profile – A Strategic Development Process Tool, US Food and Drug Administration, Washington.
7.) European Parliament and Council 2003.Commission Directive 2003/94/EC of 8 October 2003: Laying Down the Principles and Guidelines of Good Manufacturing Practice in Respect of Medicinal Products for Human Use and Investigational Medicinal Products for Human Use Off. J. European Commun.:22-26.
8.) European Parliament and CouncilCommission Directive 91/412/EEC of 23 July : Laying Dow n the Principles and Guidelines of Good Manufacturing Practice for Veterinary Medicinal Products Off. J. European Commun. of 17 August1991:70–73.
9.) 2002. Warning Letter: Automatic Liquid Packaging, Inc., US Food and Drug Administration.
10.) ICH Q9 2006. Quality Risk Management Step 4. Fed. Reg. www.ich.org/LOB/media/MEDIA1957.pdf 71:32105-32106.
You May Also Like