Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
Pharmaceutical companies of all sizes outsource at least some quality control (QC) testing to contract analytical testing laboratories. Virtual and smaller companies may not have the staff to conduct such testing, whereas mid- to large-size companies may outsource testing that they do not wish to perform in-house. In the relationship between a pharmaceutical company and its outsourcing partner, each partner has clearly delineated responsibilities, both business and compliance related. In May 2010, we discussed a contractee’s (contract giver’s) responsibilities (1). Here, we focus on those of the contractor (contract acceptor), limiting our attention to responsibilities in the contractee– contractor relationship. Neither discussion addresses purely business-related concerns such as revenue growth, development and retention of staff, and shareholder reward.
Compliance
The laboratory to which a pharmaceutical company outsources QC testing in effect becomes an extension of that company’s own QC organization. Its compliance requirements must therefore be matched by those of the contractor. In most cases, therefore, the contract testing organization must meet good manufacturing practice (GMP) requirements. For a testing organization, that generally entails clearly defined quality systems, including sample chain of custody, organization and personnel, buildings and facilities, equipment, control of components, laboratory controls, change control, and records and reports.
Evidence of a sufficient level of GMP compliance can be proven by the results of regulatory inspections, especially those conducted by the US FDA and the EMEA. With the former, satisfactory performance is evidenced by the absence of an unresolved FDA Form 483 (“Notice of Inspectional Observations”). The EMEA recognizes satisfactory performance by issuing a GMP certificate. Regular compliance visits conducted by sponsoring pharmaceutical companies contribute to a contract testing organization’s ongoing steady-state level of GMP compliance. The nearly continuous flow of inspectors through a laboratory certainly provides ample opportunity for continuous improvement in compliance.
Long-Term Partnerships
When a pharmaceutical company decides to outsource QC work, it selects a contract laboratory on the basis of several criteria (1,2,3). Aside from compliance, method, and quality considerations, the sponsoring company must have assurance that outsourced methods will remain available at a contract laboratory over the long term. So the contractor must be financially secure and willing to commit to maintaining trained staff, secured facilities, reagents, and calibrated equipment necessary for performance of those methods for as long as they are requested. Should a contractor determine that it is not in its own best interest to maintain a given assay in its portfolio, sponsor companies using that method must be given sufficient advance notice to pursue other options.
Responsiveness and Adherence to Quality Agreements: A contract testing laboratory must make itself available to a sponsoring company’s contacts. Poor responsiveness endangers the partnership. So it is advantageous for a contractor to set up a single contact for each client. That contact may better reside within a client services or program management group than in the testing laboratory itself.
A contract testing laboratory’s contacts and responsibilities are specified within its quality agreement with each sponsoring company. The content of that agreement is described elsewhere (1,3). A quality agreement specifies clearly (from the sponsor’s point of view) the contract laboratory’s responsibilities in quality systems; compliance; equipment, reagent, and method characterization/validation; data recording and archival; sample receipt, storage, and retention; interactions with client and regulatory inspectors; conduct of investigations into unexpected and out of specification (OOS) results; and change control. During contract laboratory oversight, sponsor companies typically evaluate a contractor on the basis of its performance against the terms of the quality agreement. A sponsor can expect to address deficiencies in its periodic compliance audits of the contractor.
Organization and Personnel
Contract testing laboratories are expected to maintain a high caliber of scientific expertise in their staff who direct assay performance and operators who conduct assays. This level of expertise will be called upon in the event of unexpected or OOS results. Rapid troubleshooting of problems that invalidate assays and identification of OOS root causes require a high degree of expertise with a given assay system. In addition, scientific credibility comes into play for assay designs complicated by factors not normally encountered (e.g., viral testing for infectious viral vaccines or oncolytic viruses).
In some cases, scientific expertise in a specific method is reflected by participation of contractor staff in national/international consortia establishing or revising guidance or standards pertaining to that method (e.g., US Pharmacopeia, European Pharmacopoeia, American National Standards Institute, and the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use). Accessing this level of expertise is always beneficial to a sponsoring company because it can lend a higher degree of credibility to the work being performed.
In addition to scientific expertise, a robust quality assurance (QA) unit is essential to a contract laboratory’s success. Responsibilities of the QA unit — as well as management’s responsibilities and commitment to quality — should be clearly defined and described in documents such as a quality manual or quality policy (4). Personnel should be made aware of such documents, and proof of training on them should be made available to sponsor companies during audits.
Typical QA responsibilities include review and approval of procedures such as standard operating procedures (SOPs) and analytical methods, as well as validation and transfer protocols and reports. QA also participates in execution of nonconformances, deviations, and OOS investigations; approval of change control records; and hosting of regulatory inspections and client audits, including follow-up and closure. In some companies, QA staff are involved in routine data review, which is good practice. At a minimum, QA should review and approve certificates of analysis before their issuance to clients. Management awareness of quality issues (e.g., nonconformances, OOS results, and invalid assay trends) is a GMP requirement (5). To facilitate satisfaction of that requirement, QA representatives may compile periodic summaries and submit them to management.
A well-established training program is critical to the success of all contract laboratories. In addition to technical training, all personnel involved in testing and generation of data must be trained on GMP requirements. This includes consultants and contractors (5), those who are often overlooked by contact laboratories. Such training must be documented and available for review during regulatory inspections and client audits.
Buildings and Facilities
GMP requirements for facilities must be satisfied, too. The security of laboratory spaces is ensured by batch-controlled access, keys, and/or pass codes. If laboratory spaces are shared among clients, it is important to ensure that access to those areas is limited to appropriate personnel — or that security for the entire laboratory meets requirements of the tightest security use. Limited space is often an issue for most contract laboratories. But warehouse areas, laboratory supply storage rooms, and other auxiliary areas should be clearly defined and separated from testing areas. Laboratory water must satisfy the requirements of and be tested according to the USP monograph for purified water (6).
Laboratory facilities may have very specific requirements depending on the type of testing performed in them: chemical, microbiological, viral, and so on). In some cases, especially for microbiological laboratories, testing may need to be performed in a classified environment. Specific cleaning and environmental monitoring may be required in such cases (7). All programs supporting laboratory testing facilities should be described in GMP documents.
Equipment: As with manufacturing equipment, laboratory equipment is subject to predefined maintenance (including cleaning and sterilization as necessary), calibration, and change control requirements. A well-described, holistic approach to laboratory equipment management should be in place (8). Equipment logbooks need to be maintained and available for review during regulatory inspections and client audits.
Control of Components: Selection and adequate quality control of reagents (including raw materials, cells, controls, and standards) required during performance of a method are also contractor responsibilities. GMPs require receipt, quarantine, and release of such reagents according to predefined specifications. Beyond this, contractors need to establish expiration dates for reagents, justify their assignment, and ensure that reagents are not used past their expiry dates. Appropriate storage conditions (temperature and humidity) must be available for all reagents. Standards and control articles used in analytical methods must be appropriate for each method and must be identified adequately.
Laboratory Controls
A contract testing organization needs to ensure that its methods comply with the appropriate compendia (e.g., US Pharmacopeia, European Pharmacopoeia, Japanese Pharmacopeia), regulations (e.g., 21 CFR Part 211 and EU GMPs), and/or regulatory guidances (e.g., points to consider and ICH harmonized tripartite guidelines) as stated in method documentation (e.g., SOPs, technical specifications, protocols) and represented to clients. To ensure that compliance is ongoing, a contract laboratory should follow an internal process for compendial surveillance and must have access to compendial updates (e.g., Pharmacopeial Forum, PharmEuropa, JP Forum).
Method validation for noncompendial assays used in GMP-compliant testing is the responsibility of the contract laboratory (9). Its failure to provide evidence of such method validation for a GMP assay may trigger a Form 483 from the FDA. Method verification is required for compendial assays (10). Although it is the client’s responsibility to submit samples of test material for method verification, a contract testing laboratory should as part of its testing service remind clients of this responsibility and offer suitable verification procedures. Similarly, it is the client’s responsibility to commission and provide test samples for method qualifications required with noncompendial methods (1), although contract testing laboratories have on occasion received Form 483s after failing to properly qualify a commercial product for a specific test performed for a client.
When a sponsor performs method validation is performed, method transfer studies are needed to ensure that the contract laboratory can execute the related testing (11). The contractee and contractor may have to ensure that noncompendial GMP testing performed on a commercial product is properly qualified as suitable for that product — and that such qualification is documented in a report that can be provided to a regulatory agency upon demand.
Change control over assay documentation is another responsibility of the contract testing laboratory. A pharmaceutical company outsourcing its QC testing will have filed the contractor name and contractor method within the chemistry, manufacturing, and controls (CMC) section of its investigational new drug (IND) or biologics license application (BLA). The company is then obliged to inform the regulatory agency of significant changes in a given analytical method (12). Those cannot occur if a contract testing laboratory does not document procedural changes in its own change control system and/or the laboratory fails to notify a sponsoring company of the change.
Records and Reports
A contract testing laboratory must keep accurate data records and archives. Its ability to rapidly access those data — and the ability of the data to withstand regulatory scrutiny during an inspection — are great concern to sponsor companies. The information itself may be addressed by the sponsor either on completion of initial studies performed by the contractor or during periodic compliance audits. The contractor’s archiving practices are evaluated during initial assessment of quality systems and typically examined during periodic compliance audits of the contractor by the pharmaceutical company.
Defining the Relationship
A contract testing laboratory performing QC work outsourced from a pharmaceutical partner becomes an extension of the sponsoring company’s QC department. For this reason, regulatory agencies and sponsor companies regularly scrutinize contract testing organizations. Compliance expectations to be met are determined by the compliance level of the sponsoring company and the status of its test material. Best-in-class contract testing laboratories meet each of these expectations (Figure 1).
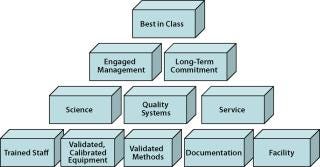
Figure 1: ()
The long-term relationship between a sponsor company and its testing partner depends in large part on the degree to which the outsourcing partner meets its responsibilities. Failure to adhere to such responsibilities may result in compliance failures that threaten the success of a sponsoring company’s product development and registration.
We have provided our opinions based on experiences in contract testing organizations and sponsoring companies. This information can provide a starting basis for building an effective and successful contractee– contractor partnership but should not be considered the definitive word on these subjects. As always, it is the regulatory agencies themselves who provide the ultimate guidance.
About the Author
Author Details
Corresponding author Raymond W. Nims, PhD, is a consultant for RMC Pharmaceutical Solutions, Inc., 2150 Miller Drive, Suite A, Longmont, CO 80501; 1-303-776-5200, fax 1-303-776-5201; [email protected]; www.rmcpharma.com. Elizabeth Meyers, MS, is a compliance manager at Amgen, Inc.
1.) Nims, RW, and E. Meyers. 2010. Contractor Responsibilities in Outsourced Pharmaceutical Quality Control Testing. BioProcess Int. 8:26-33.
2.) Griff, I, and RW. Nims. 2006. Quality Control Testing: How to Select and Work with a Contract Testing Organization. BioProcess Int. 4:S30-S32.
3.) Strauss, M, and J. Novak. 2009. Selection and Oversight of Domestic and International CMOs. BioProcess Int. 7:24-31.
4.) ICH Q10 2009. Pharmaceutical Quality System. Fed. Reg. www.ich.org/LOB/media/MEDIA3917.pdf 74:15990-15991.
5.) 2009.Current Good Manufacturing Practice for Finished Pharmaceuticals Code of Fed. Regulations Title 21, US Food and Drug Administration, Rockville.
6.). 2010.USP General Notices and Requirements Applying to Standards, Tests, Assays, and Other Specifications of the United States PharmacopeiaUSP 33–NF 28, US Pharmacopeial Convention, Rockville.
7.) Sutton, S. 2007.Chapter 3: Laboratory Design ConceptsPharmaceutical Quality Control Microbiology: A Guidebook to the Basics, David Health International Publishing, LLC, River Grove.
8.) 2010.General Chapter Analytical Instrument Qualification“USP 33–NF 28, US Pharmacopeial Convention, Rockville.
9.) ICH Q2 (R1) 1995.Validation of Analytical Procedures: Text and Methodology Fed. Reg.:11260.
10.) 2010.General Chapter Verification of Compendial ProceduresUSP 33–NF 28, US Pharmacopeial Convention, Rockville.
11.) Quattrocchi, O. 2009. Transfer of Analytical Procedures: A Proposal for a New General Information Chapter (A Stimulus Article). Pharmacopoeial Forum 35.
12.) 2009.Supplements and Other Changes to an Approved Application Code of Fed. Regulations Title 21, US Food and Drug Administration, Rockville.
You May Also Like