Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
June 1, 2008
Readers of our February 2008 article “Construction and Start-Up Costs for Biomanufacturing Plants: Canadian Case Studies in the Cost of Regulatory Compliance” may have noticed something missing (1). Two somethings, in fact: First, biographical information for coauthor Agnès Coquet was not listed at the end of the article. She is manager of analytical development for Debiovision Inc. of Montreal, Quebec, in Canada; 1-514-842-9976, ext. 104; acoquet@debiopharm. ca. Second, “Table 1” was called out on the fourth page of the article (p. 20 of the February issue), but that table was not included in the layout. We apologize for any inconvenience or confusion that may have resulted. The online archival version of the article is now correct (www.bioprocessintl.com/default.asp?page=articles&issue=2%2F1%2F2008).
Table 1: Multiplying factors used by three consulting firms (cost/ft2 of each area)
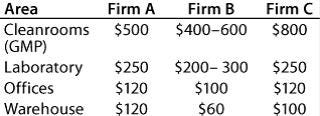
To make up for the “snafu,” I wanted to offer in this space all the tables the authors included with their original paper, not just the one that was called out in the text. Here on this single page, they represent well the major point of the article: GMP compliance costs money!
Three consulting firms provided estimates on the cost of building a biotech facilty. Table 1 shows their suggested costs per square foot of different types of space. Then three case studies were provided to illustrate differences among different types of companies. Table 2 shows facility costs for Company A, a regenerative medicine company. Table 3 shows that company’s start-up costs. Table 4 and Table 5 show facility and start-up costs, respectively, for a supplier of natural marine collagen (Company B). And Table 6 and Table 7 show facility and start-up costs for a maker of recombinant protein biopharmaceuticals using transgenic plants (Company C). All these companies are located in Canada.REFERENCE
Table 2: Facility costs for Company A
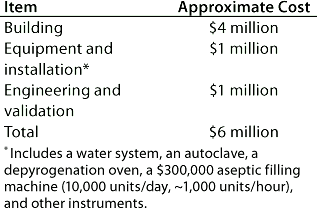
Table 3: Start-up costs for Company A
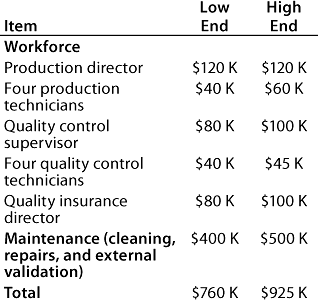
Table 4: Facility costs for Company B
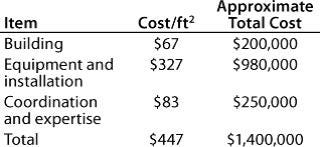
Table 5: Start-up costs for Company B
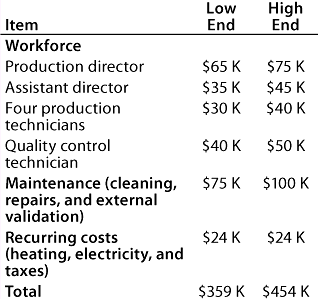
Table 6: Facility costs for Company C
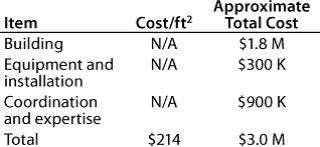
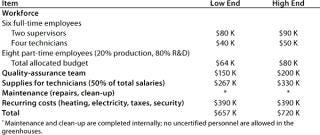
Table 7: Start-up costs for Company C
1.) Denault, J-F, A Coquet, and V. Dodelet. 2008. Construction and Start-Up Costs for Biomanufacturing Plants: Canadian Case Studies in the Cost of Regulatory Compliance. Bio Process Int. 6:14-23.
You May Also Like