Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
August 11, 2016
Sponsored by PendoTECH
Jim Furey (general manager, PendoTECH) BPI Theater @ INTERPHEX, April 26, 2016 11:30 am–12:00 pm
Located in Princeton, NJ, PendoTECH was founded in 2005. The company develops equipment for single-use systems and promotes the concept of automation. It produces sensors, monitors, transmitters, and standard process control systems with the ability to collect and store data, as well as customized solutions. PendoTECH staff have many decades of experience in product development, project management, embedded software development, graphical-interface programs, and mechanical engineering.
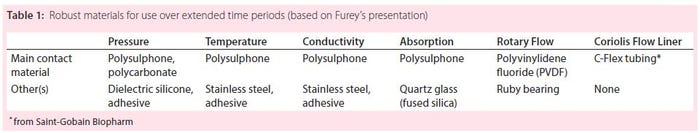
Biopharmaceutical manufacturers use sensors primarily to measure pressure, temperature, conductivity, absorbance, turbidity, and flow with applications in both upstream and downstream processing. When it comes to single-use sensors, they have questions: e.g., “Will it self-destruct?” In this case, disposabiliy is related to low cost. The single-use component is minimized by design so that most of the electronics can be recycled, which is also facilitated by minimized product-contact components. These sensors are durable and can be used for continuous processing.
PendoTECH ensures that its sensors will perform well with single-use systems. They are made in a clean environment of correctly certified contact materials. Data collected are accurate and repeatable. The sensors are chemically compatible with fluids used in processing and can withstand sterilization processes (extreme temperatures and gamma radiation). Because they meet regulatory standards, the sensors can be integrated into any control system (local or transmitted to a higher level). They are made up of both physical and electronic elements and provide both analog (local) and digital (transmitted) outputs.
Furey emphasized that the single-use sensors are robust, that the technology can be used in good manufacturing practice (GMP) processes, and that qualification data are available from the supplier. Users can perform real-time monitoring of process performance and evaluate new production platforms. For more information, articles and data are available online at www.pendotech.com.
Questions and Answers
Do you make metabolic sensors?
Not specifically, but PendoTECH partners with other companies to integrate their sensors into its product line and thus make those available.
What about pH sensors?
The company is not producing these right now.
How do your sensors work in low-flow situations?
It is the Achilles heel of in-line sensors: They have trouble with low flows. PendoTECH’s rotary flow meter can measure ≥60 mL/min flows.
How long does the supplier take to develop new products?
That can take a year to many years, depending on the product and associated failure rates and how readily the company can find the needed expertise.
Further Reading
Anarelli D, et al. Measuring Pressure at Very Low Levels with High Accuracy in Single-Use Systems. BioProcess Int. 13(3) 2015: S44–S47.
Bink LR, Furey J. Using In-Line Disposable Pressure Sensors to Evaluate Depth Filter Performance. BioProcess Int. 8(2) 2010: 44–48.
Kluck B, et al. Streamlining Downstream Process Development. BioProcess Int. 9(6) 2011: 54–60.
Renaut P, Annarelli D. Evaluation of a New Single-Use UV Sensor for Protein A Capture. BioProcess Int. 11(2) 2013: 48–51.
Checklist for Single-Use Sensors |
You May Also Like