Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
August 10, 2016
Sponsored by Gyros
 Host cell protein (HCP) levels in biologics are on the critical path for assessing product quality, and they pose a serious risk to drug safety. The challenge is to accurately quantify a complex mixture of HCP impurities, which vary in properties and abundance depending on cell line, media, and process parameters. HCP immunoassay analysis is based on polyclonal antibodies raised against HCPs from nontransfected cell lines.
Host cell protein (HCP) levels in biologics are on the critical path for assessing product quality, and they pose a serious risk to drug safety. The challenge is to accurately quantify a complex mixture of HCP impurities, which vary in properties and abundance depending on cell line, media, and process parameters. HCP immunoassay analysis is based on polyclonal antibodies raised against HCPs from nontransfected cell lines.

Automated immunoassays with high-throughput (Gyrolab xP workstation, left) or single-CD configuration (Gyrolab xPlore, right)
How well a particular HCP assay recognizes all proteins depends on how well the antibodies match the HCP composition, their abundance, and their affinities. To cover the diversity of HCP mixtures from different bioprocesses, Gyros has developed a panel of five Gyrolab CHO-HCP kits to increase the possibility of finding the assay that best matches a specific CHO-K1 process. A scouting kit simplifies selection of the optimal generic kit with broader dynamic ranges, high sensitivity, and time to results in about an hour.
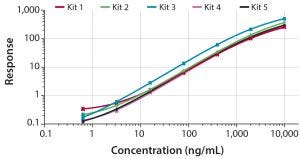
Figure 1: Dose response curves for five kits developed for quantification of CHO HCP in bioprocess samples covering a quantifiable range from 1–3 ng/mL up to 8,000 ng/mL
Gyros’ CD-based nanoliter-scale immunoassay technology improves productivity by providing rapid assay development time, automation, high throughput, and a broad dynamic range that minimizes dilutions and repeats.
To achieve that, Gyrolab™ systems perform automated immunoassays within microfluidic structures in a compact disc (CD) format. Each structure on the Bioaffy CD consists of a 15-nL affinity column prepacked with streptavidin-coated particles, supporting a variety of assay types, including sandwich and indirect antibody assays. The affinity flow-through format minimizes interference and eliminates incubations for rapid time to results. Microfluidics ensure controlled flow of capture reagents, sample, and detection reagents to process all samples within a single CD in parallel.
Gyrolab HCP Kits: Broader Dynamic Range and Robustness
The five kits were validated according to conventional performance parameters, including LOD, LLOQ, ULOQ, precision, linearity, and spike recovery. All kits demonstrated LLOQ in the range of 1–3 ng/mL, and the ULOQ was 8,000 ng/mL of CHO HCP.
Gyrolab HCP Kits for Improved HCP Analysis |
Gyrolab systems with Gyrolab CHO-HCP kits simplifies HCP kit selection while improving performance and productivity for downstream purification |
Selection of Five CHo-HCP Kits
|
Easy-to-Use Kits
|
Performance
|
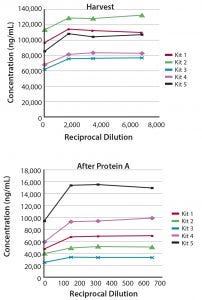
Figure 2: Measurement of CHO-HCP during a purification process; (top) harvest and (bottom) after protein A) of a human IgG1 antibody produced in CHO cells were evaluated for CHO HCP in the linear range of Gyrolab CHO-HCP Kit 1–5.
Selecting the Best Kit to Monitor HCP Levels in Bioprocess Samples
HCP levels were measured at various stages over 12 days during the purification of a recombinant human IgG1 antibody produced from a 2-L-scale cell culture of a CHO cell line. The five Gyrolab CHO-HCP kits had similar abilities to detect HCP impurities in the final product (not shown), but because processes can vary between rounds of cell culture, the optimal kit was selected based primarily on the results for HCP impurities in harvest and post-protein A samples (Figure 2). Gyrolab CHO HCP Kit 2 or 5 appears to be the best choice for this particular process.
QuantifyinG CHO HCP impurities in Pharmaceutical-Grade Drugs
Six drug products manufactured in CHO cells were evaluated for the presence of CHO HCP antigen in the linear range of the assays. Three drug products contained low concentrations of CHO HCP (<1 ppm). The remaining three drug products (B, D, and E) contained more substantial amounts of CHO HCP, as indicated in all five CHO HCP kits (Figure 3). Different kits, however, demonstrated different abilities to quantify CHO HCP reproducibly, and ranking of kits based on the outcome of these three drug products showed that each drug has its own best kit. In one case, the CHO HCP concentrations determined differed by a factor of approximately 30 when using the different kits. When Kit 2 was used, the CHO HCP impurity level greatly exceeded the acceptable level of 100 ppm.
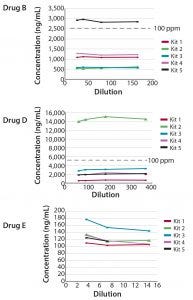
Figure 3: Selected drug products evaluated for CHO HCP in the linear range of Gyrolab CHO-HCP Kit 1–5
The Future of HCP Analysis
Host cell proteins are major impurities that can affect the safety of medicines derived from cell-based production systems. The absence of HCPs will inevitably be included among critical quality attributes (CQAs) of monoclonal antibodies. But accurately quantifying a complex mixture of HCPs is challenging because their properties and abundance vary depending on cell line, media, and process parameters. Immunoassays based on polyclonal antibodies that quantitate “total” HCPs play an important role because of their high throughput and potential for automation.
Helena Nilshans is product manager at Gyros AB, Uppsala, Sweden; [email protected]. Gyros is now part of Gyros Protein Technologies. For more information about the merger, visit www.gyrosproteintechnologies.com.
You May Also Like