Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.

Industrial-scale production of pure monoclonal antibodies (MAbs) customers require an overall highly efficient affinity resin with a high binding capacity, high alkaline stability, good elution profile, low nonspecific binding, and large-scale column operation. Kaneka has developed a novel protein A ligand and immobilized it to highly cross-linked cellulose beads to totally satisfy all these requirements. Here, we demonstrate the performances of the Kaneka KanCapA using MAbs.
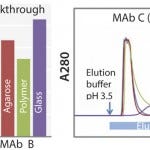
FIGURE 1: Dynamic binding capacity (for MAb A and B) and elution profile (for MAb C) of KanCapA compared with commercially available protein A resins; 5% breakthrough is determined at 6 min of residence time (left); 5 mg/mL-resin of IgG (VH3 subfamily) is loaded and elution is performed at pH 3.5. Strip solution is 1 M Acetic acid (right).
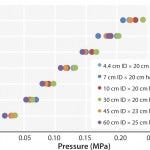
FIGURE 2: Pressure/flow rate characteristics of KanCapA packed into various column sizes; mobile phase is water. The pressures generated by packed beds are calculated by subtracting the system pressure from total pressure.
Mild pH Elution and High-Binding Capacity: The elution profile of a protein A column is one of the most critical factors in resin selection. For example, the VH3 subfamily of MAbs requires a lower elution pH than other types, because it contains an additional protein A binding site in the Fab region. However, it is well-known that lower elution pH leads to a higher formation of aggregates. Accordingly, Kaneka KanCapA ligand has been designed to remove this undesired Fab binding and make the MAb elution in mild acidic conditions possible (Figure 1, right). This considerably simplifies the process development of a MAb purification platform.
Moreover, binding capacity tests for MAbs revealed that Kaneka KanCapA has a high binding capacity comparable with that of commercially available agarose medium (Figure 1, left) Additionally, Kaneka KanCapA has an excellent alkaline stability and can be used up to 300 cycles with 0.1M sodium hydroxide as CIP solution (data not shown).
High Flow Rate Operation and Easy Scale Up: Figure 2 and Table 1 show pressure/flow-rate characteristics and packing efficiency of the packed columns, from 4.4 cm to 60 cm i.d. Kaneka KanCapA can be packed using flow and axial compression packing methods from pilot to large scale and the columns show excellent pressure flow characteristics with good column performance.
Conclusions
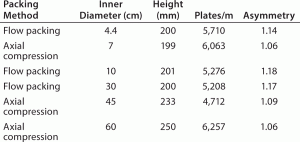
TABLE 1: Column performance of the packed columns evaluated by pulse injection; sample/eluent = 1%(v/v) acetone/water or 0.5 M NaCl/0.1M NaCl; injection vol = 1% of column volume; flow rate = 60 cm/h
Kaneka KanCapA demonstrates high efficiency in the MAb capture step, making it an easy-to-use and trustable protein A resin recommended for your MAb purification platform. Its excellent performance has been already acknowledged and some global pharmaceutical companies have already adopted Kaneka KanCapA for clinical-phase drug production. For more information, please visit our website at www.bioseparation.kaneka.com.
Koji Iritani ([email protected]) and Hideo Kitahashi
are managers, and Takumi Sato and Keisuke Bando are marketing in the healthcare group, biochemical and medical business development division, at Kaneka Corporation, 2-3-18 Nakanoshima, Kita-ku Osaka 530-8288, Japan.
You May Also Like