Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
August 15, 2014
 Fujifilm Diosynth Biotechnologies (FDB) leverages over 15 years of experience in bioprocess development and manufacturing to successfully deliver robust process designs to its clients. FDB has executed a series of innovation programs to enable rapid development of cell culture processes, including the coming launch of a new Chinese hamster ovary (CHO) expression system. This application note describes how outputs from media screening and upstream process development innovation programs were applied to provide significant productivity and process robustness improvements to an early stage client program.
Fujifilm Diosynth Biotechnologies (FDB) leverages over 15 years of experience in bioprocess development and manufacturing to successfully deliver robust process designs to its clients. FDB has executed a series of innovation programs to enable rapid development of cell culture processes, including the coming launch of a new Chinese hamster ovary (CHO) expression system. This application note describes how outputs from media screening and upstream process development innovation programs were applied to provide significant productivity and process robustness improvements to an early stage client program.
Results
Toolbox Media/Feed Screening: FDB developed a toolbox of high-performance basal media and feeds by screening numerous commercial and prototype media with diverse CHO cell lines.
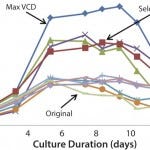
FIGURE 1: Cell growth trends with various basal and feed media
combinations
In this case study, 10 basal media and feed media combinations were selected for fed-batch production trials in shake flasks. Varying degrees of cell growth (Figure 1) and titer (not shown) were observed. Even though the highest cell growth condition provided the highest product titer, product quality was compromised. The media/feed combination with the next highest titer and robust growth met product quality standards and was selected for process parameter development.
Process Development: Selection of a high-performance basal media and feed for a given CHO cell line provides a foundation upon which a process can be further developed. In this case, FDB development experience was leveraged to identify a subset of key process parameters to study with the goal of significantly improving titer and process robustness, while maintaining or exceeding product quality standards.
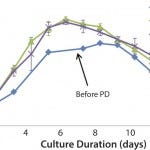
FIGURE 2: Cell growth trends following process parameter development
Figure 2 shows that cell growth in 10-L and 200-L single-use bioreactors was improved beyond the toolbox media shake flask results. In addition, the product titer was dramatically improved over three fold at both the 10-L and 200-L scales. The good agreement between the 10-L and 200-L bioreactors shows the scalability of the FDB single-use platform process. Agreement among the center-point 10-L bioreactor cultures indicates that the process is robust and ready for scale-up to manufacturing.
Conclusions
FDB applied cell culture expertise in basal media and feed screening and process development to achieve over three-fold titer increases with improvements in process robustness and product quality. Pairing this process design capability with FDB’s single-use manufacturing platform allowed FDB to deliver a customized and reliable cell culture manufacturing process rapidly and cost-effectively.
Stewart McNaull is director, development and technical services ([email protected]), Carrie Weymer is scientist II, upstream development, Mark Patton is scientist I, upstream development, Meredith Jones is senior scientist, upstream development, and Tim Hill is director, upstream development.
You May Also Like