Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.

Production of biologics — whether for clinical development or large-scale manufacturing campaigns intended to be converted to final drug product — often involves frozen storage. It provides manufacturing process flexibility while enabling long-term product stability. Products are frozen and stored using a number of technologies: stainless-steel vessels, bottles, carboys, and disposable bags. Although single-use freezing bags are a common choice for storing biologics, challenges with durability persist.
Single-use bags intended for freezing and storage are often made with films using ethylene-vinyl acetate (EVA) and/or low-density polyethylene (LDPE) polymers, with product routinely blast-frozen and stored to −30 °C in a cold-storage warehouse. During freezing and transport, the bags will typically experience temperatures well below −30 °C ranging from −50 °C to −80 °C. Under these conditions, the bags have to endure a wide range of stresses (film brittleness, volume expansion, and so on) that impact integrity. Although improvements have been made — mainly by adding a specifically designed secondary protective freezing/storage shell — film fractures and breaks are still common with commercially available bags.
The new single-use Freeze-Pak™STS (FP-STS) frozen storage and transport solution containers are manufactured using a unique polyolefin monolayer film designed for freezing applications that remains flexible to temperatures as low as −196 °C. Maximum fill volumes range up to 17 L, and a “boat” port design offers a wide range of tubing configuration options to support process variation. FP-STS bags deliver the flexibility and durability required for frozen storage and transport of biopharmaceuticals.
The purpose of this study was to validate the new Freeze- Pak™STS bags (Charter Medical, Ltd., Winston-Salem, NC) for freezing and storage. These results demonstrate that FP-STS freezing bags can be used for freezing and subsequent storage to −80 °C.

Methods
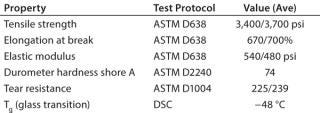
Film Properties: Basic film properties for the FP-STS polyolefin film were determined using standard validated methods. Table 1 lists those properties and associated test protocols.
Table 1:
Freeze Bag Validation Testing: To validate the FP-STS containers for their intended use, a selection of tests were performed: physical integrity, freezing integrity, and robustness.
Physical Integrity: Before freezing, bags were evaluated for potential leaks or failures using an unconstrained pressure leak test (1.0 psi). Additional testing was performed by filling 2-L and 20-L bags to nominal fill volumes and hanging (with contents in a liquid state) for a minimum of 72 hours.
Freezing Integrity: 20-L bags (a worst case) were filled to at least 85% of nominal capacity and frozen to −80 °C. Bags were then hung and thawed for a minimum of 72 hours, and this process was then repeated (for two freeze–thaw cycles per bag).
Additional robustness testing was achieved by performing a drop test filling 1-L, 5-L, and 20-L bags to 85% nominal volume before dropping them. Bags were then frozen to −80 °C, thawed, and dropped a second time. Drop heights were 6 feet, 3 feet, and 2 feet for the 1-L, 5-L, and 20-L bags, respectively. For all testing, the bags, ports, tubing, and connectors were assessed for possible damage or leaks. No damage or leaks were deemed acceptable.
Data Summary: This study introduces the new Freeze-Pak™ STS disposable freezing bags and describes the validation testing performed to support the efficacy and utility when frozen storage of products is required. Before building a bag intended for storing products frozen, a robust, flexible film designed for freezing is necessary. The testing and data shown in Table 1 represent some key physical aspects of the polyolefin film used for these bags. The film combines both durable (hardness and resistance to tearing) and flexibility (elongation, elastic modulus) features along with a low Tg (−48 °C) temperature.
Table 2:

A New Solution to an Ongoing Challenge
The Freeze-Pak™ STS disposable freezing bags are designed to support frozen storage product needs. The validation studies support the efficacy and durability of the FP-STS containers to storage temperatures as low as −80 °C. Supporting studies are currently in progress to validate secondary storage and transport container options to be used with FP-STS bags. Those data will be reported in a separate study. In conclusion, the single-use Freeze-Pak™ STS containers from Charter Medical, Ltd. offer a new durable yet flexible freezing, storage and transport solution.
About the Author
Author Details
Dominic Clarke, PhD, is global product manager for bioprocessing and cellular therapy ([email protected]), and Jared Ragone is a new product development engineer at Charter Medical, Ltd.,
3948-A Westpoint Blvd, Winston-Salem, NC 27103, www.Chartermedical.com. Freeze-Pak™ is a trademark of Charter Medical, Ltd., Winston Salem, NC. Freeze-Pak™ STS bio-containers are intended for frozen storage and transport.
You May Also Like