Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
August 1, 2013

One of the major bottlenecks in the production of biopharmaceuticals is the efficient expression of therapeutic proteins in microbial or mammalian cells. The Escherichia coli pAVEway™ expression system described here has been developed to ensure high product titers and efficient scale up to GMP manufacture while minimizing many common issues seen in other expression systems, such as “leaky” expression (expression of recombinant protein in the absence of inducer).
How It Works
The use of a number of powerful E. coli RNA polymerase promoters — such as T7A3, λpL, and tac — opens up a large host range in comparison with the popular T7 system that is limited to hosts carrying the λDE3 prophage. Control over basal expression is provided by the use of perfectly palindromic lac operator sequences (Figure 1). This high level of control is extremely important in large-scale (≥5 L) fermentations because it allows high biomass accumulation prior to induction, which can be helpful when expressing proteins that are potentially toxic to E. coli. The ability to effectively “switch off” protein expression until induction ensures that all cells are capable of protein production. For an expression system used in large-scale manufacture, this is a highly desirable characteristic and demonstrates control over the process. This also enables a generic high–cell-density fermentation protocol to be used for any protein, with no specific optimization required, which allows gene to fermentation in as little as five weeks.
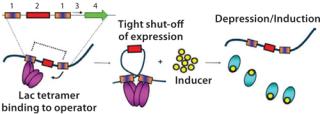
The rate of target gene transcription in the pAVEway™ system can be controlled by varying the concentration of inducer (IPTG). This can be utilized in situations where the maximum rate of expression may not be needed for optimal accumulation of the appropriate form of the protein. For example, it may be beneficial to express a recombinant protein targeted for secretion or soluble intracellular expression slowly, so that the host secretion machinery or folding capacity is not overloaded as this can greatly reduce the growth and productivity of recombinant cells. Different combinations of the two promoter region components lead to a range of pAVEway™ vectors with expression kinetics that can be tailored to the requirements of a specific protein and its production route. When combined with generic high–cell-density fermentation protocols, high titers of a diverse range of biopharmaceuticals — from viral and bacterial proteins through to complex mammalian proteins such as growth factors, cytokines, and antibody fragments — have been produced.
Since the pAVEway™ system was launched in 2007, a wide experience base has been generated by covering such a diverse range of molecular types. This confirms the ability of the system to generate high-productivity E. coli strains with excellent scalability for biopharmaceutical production.
Continuing development of the pAVEway™ system will provide additional benefits for users. The presence of antibiotic in fermentation media is becoming an increasing regulatory concern, so the recent development of an antibiotic-free pAVEway™ system without compromising product yield highlights its versatility.
About the Author
Author Details
Jonathan Pointon, PhD, is principal scientist at Fujifilm Diosynth Biotechnologies, Billingham, UK site. Fujifilm Diosynth Biotechnologies, 101 J. Morris Commons Lane, Morrisville, NC 27560; 1-866-762-6259; [email protected]. pAVEway™ is a trademark, the property of Fujifilm Diosynth Biotechnologies UK Limited.
You May Also Like