Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.

Current downstream processing strategies for recombinant proteins often require multiple chromatographic steps, which may lead to poor overall product yields. Product purification can be especially difficult when a target protein displays reduced stability, has isoforms or misprocessed variants, or needs to be purified from a complex mixture containing a high level of impurities. Through highly specific antibody–antigen based interactions, affinity chromatography brings significant technical advantage to these protein purification challenges.
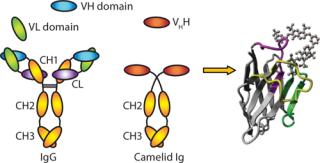
Developed to tackle such challenges, CaptureSelect™ technology is based on chromatography matrices functionalized with highly specific, camelid-derived but yeast-expressed, single-domain antibodies. With a single peptide chain of ~110 amino acids (MW 12–15 kDa) comprising a single monomeric variable domain of a camelid heavy-chain–only antibody (VHH) (Figure 1), these fragments are significantly smaller and more stable than conventional antibodies. These properties open a range of affinity chromatography applications: from purification of recombinant proteins and specific antibodies to high-speed quantitation of biomolecules. Such applications are described herein.
Purification of a Recombinant Protein – Follicle Stimulating Hormone (FSH)
The CaptureSelect™ follicle stimulating hormone (FSH) affinity resin is specifically designed for purification of FSH in bioprocess applications. This novel affinity ligand recognizes the intact FSH molecule without binding its individual α and ß subunits. This enabling affinity resin is suitable for purification of intact FSH directly from complex materials. High product purity and yield with neutral pH elution can be obtained in a single purification step, demonstrating the benefit of affinity capture chromatography.
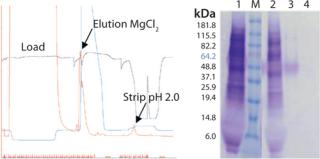
Method for Purification of FSH from Feedstock: CaptureSelect™ FSH affinity resin was packed into a column. The column was equilibrated with 10 column volumes (CV) of 20 mM Tris, pH 7.4. CHO clarified harvest containing recombinant FSH was loaded on the affinity column at neutral pH. The dynamic binding capacity for FSH is about 3 mg/mL resin at 150 cm/h. The column was washed with 10 CV of 20 mM Tris, pH 7.4. FSH was eluted from the column using 20 mM Tris, 2.0 M MgCl2, pH 7.4 in ~3 CV. After elution, the column was stripped with 0.1 M Glycine pH 2.0 and subsequently reequilibrated. Figure 2 is an example of FSH purification.
Platform Purification of IgGs
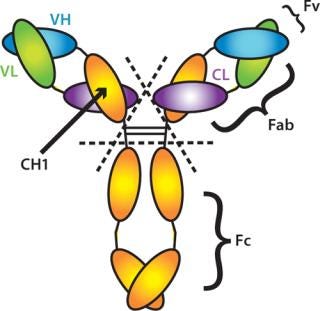
The CaptureSelect™ IgG-CH1 affinity resin recognizes the CH1 domain of human IgG (Figure 3). This unique affinity ligand enables the purification of all subclasses (1, 2, 3, and 4) of human IgG and Fab fragments, of both kappa and lambda isotypes, from any source material. Due to the human specificity of the ligand, the resin is suitable for purification of IgGs and antibody fragments even in the presence of fetal calf serum.
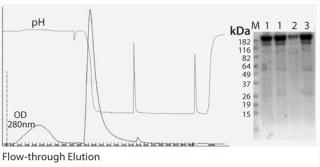
Method for Purification of IgG or IgG Fragments Using IgG-CH1 Affinity Resin: CaptureSelect™ IgG-CH1 affinity resin was packed into a column. The column was equilibrated with 10 CV of PBS, pH 7.4. Polyclonal human IgG was loaded on the IgG-CH1 affinity column at neutral pH. The dynamic binding capacity for human IgG is 15 mg/mL resin at 150 cm/h. The column was washed with ~10 CVs of equilibration buffer. The resin was eluted with 5 CV of 0.1 M glycine, pH 2.5. The starting material, flow-through, and elution fractions were analyzed using SDS-PAGE. Figure 4 is an example of Fab purification.
High-Speed Quantitation of IgGFc Fusion Proteins
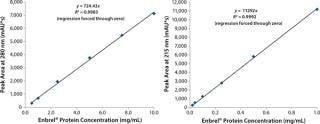
POROS® CaptureSelect™ columns contain high-performance 20-µm affinity resins, enabling rapid and precise quantitation of target biomolecules in process samples including clarified cell culture harvest and downstream product pools. Here, Enbrel® protein (an Fc anti-TNF fusion protein) is quantified during CHO cell–based production. This data set serves as a model for the capability of the entire POROS® CaptureSelect™ affinity column product line for the rapid and precise quantification of biomolecules and small-scale sample preparatrion allowing for subsequent analytical characterizat
ion. The data presented show the construction of standard curve for a POROS® CaptureSelect™ IgG Fc column application (Figure 5). The high-throughput assay is sensitive and linear, and it presents with a broad dynamic range.
Conclusion
Designed for specificity and stability, CaptureSelect™ affinity products enable a wide range of affinity applications, including laboratory- and process-scale affinity purification, rapid quantitation in discovery or throughout a downstream process, and small-scale sample preparation to support analytical characterization of biomolecules. The use of CaptureSelect™ affinity technology enables highly specific affinity binding that drives reduced cost of purification, higher quality product, and higher product yield. These features make CaptureSelect™ products a preferred tool for bioseparations, exhibiting broad applicability across a wide range of life-science applications.
About the Author
Author Details
Christine Gebski, Malcolm Pluskal, Remko Clasen, Frank Detmers, Pim Hermans, and Richard Garretson are with Life Technologies, 5791 Van Allen Way, Carlsbad, CA 92008.
You May Also Like