Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
August 1, 2012

Microbial contamination of cell cultures is a major process risk that leads to lost production batches with considerable economic impact for a biopharmaceutical manufacturer. Incidences of penetration of typical 0.2-µm sterilizing-grade membranes by mycoplasma species have led to higher-retention 0.1-µm–rated filters becoming commonplace for filtering mammalian cell culture media.
Increasing Retention without Slowing Down Processing
There are a number of 0.1-µm–rated filters available, and although many are effective at removing mycoplasma contamination, the tighter filtration membrane required is detrimental to the system flow rate. With strict time limits from the addition of the first media component to the end of filtration at media suppliers and biopharmaceutical manufacturing facilities, this can significantly increase the risk of contamination of unfiltered media.
Parker domnick hunter’s new PROPOR MR has been specifically developed using our customers’ feed streams to deliver industry leading flow rates without compromising on mycoplasma removal assurance. Combining a highly retentive 0.1-µm PES membrane (typically LRV >10 for Acholeplasma laidlawii) with an integral high-capacity PES prefilter layer, the PROPOR MR reduces media processing time to minimize contamination risks.
In addition, the optimized membrane combination increases filtration capacity and has been proven to reduce the need for separate prefilters in customer processes. For systems using cartridge filters, this reduces capital plant costs, utility requirements, and installation times. Smaller-scale media filtrations that use single-use capsule filter technology require smaller manifold designs, fewer connections and lower disposal costs.
Figures 1 and 2 show data comparing the performance of the PROPOR MR with two leading competitive mycoplasma removal filters on Tryptic Soy Broth and CHO media. Both graphs show the PROPOR MR outperforms the competitive products with respect to both flux rates and total throughput.
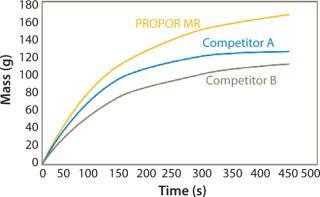
Figure 1: ()
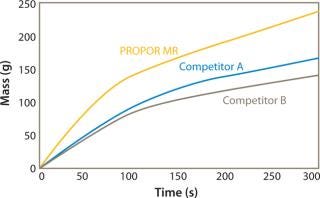
Figure 2: ()
Conclusion
Parker domnick hunter’s PROPOR MR has been demonstrated to provide class-leading flow rates without compromising on mycoplasma retention. Filtration studies on different cell culture media have shown the PROPOR MR to outperform leading competitive products providing the following benefits
Industry-leading flow rates enabling batches of media to be rapidly prepared reducing risks of contamination due to delayed processing
An integral prefilter layer reducing requirements for separate prefiltration to minimize processing costs
Validation by bacterial challenge testing with Acholeplasma laidlawii to a typical LRV of >10 offering assurance of mycoplasma retention.
About the Author
Author Details
Andrew Kelly is life sciences product manager, Parker Hannifin Manufacturing Ltd., domnick hunter Process Filtration, Europe Durham Road Birtley, Co. Durham, DH3 2SF, England, +44-191-301-4189, [email protected], www.parker.com/dhpbiopharm.
You May Also Like