Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.

Protein A chromatography is still the preferred capture step in monoclonal antibody (MAb) production because of its high selectivity and robustness, although cation-exchange (CEX) or multimode chromatography techniques are claimed as alternatives. Kaneka Protein A Cellulose increases the value of protein A affinity chromatography for MAb production.
Kaneka’s unique combination of a proprietary designed alkaline stable protein A ligand and a highly cross-linked cellulose base matrix meets customer requirements for improved performance, high binding capacity, alkaline and chemical stability for cleaning in place (CIP), mild acidic elution pH, small nonspecific binding, and scalability with good pressure flow characteristics. Kaneka’s new Protein A Cellulose is therefore a powerful tool for the improvement of your current MAb purification platform.
Dedicated Design of Protein A Ligand
The elution profile of a Protein A column at the industrial scale is one of the most critical factors. For example, the VH3 subfamily of MAbs require a lower elution pH than other types of Mab due to an additional Protein A binding site in the Fab region. It is well-known that lower elution pH leads to higher aggregates formation. Kaneka eliminated Fab binding ability to the protein A ligand and could narrow the elution pH to mild acidic range for all types of MAb.
By means of computer-aided molecular calculation, three-dimensional (3D) structural model analysis of the C domain/Fab complex, and passive screening of genetically engineered ligands with several amino acid substitutions, Kaneka created a novel protein A ligand that makes it possible to select mild acidic conditions for elution. Furthermore, Kaneka’s protein A ligand can be used under harsh alkaline conditions for CIP (see below).
Figure 1 shows a schematic representation of the new ligand construction and the improved elution profile for the VH3-encoded MAb, trastuzumab. Elimination of binding to the Fab region makes it possible to elute all types of MAbs using mild acidic conditions (pH 3.5–3.8) and simplifies the process development of a MAb purification platform.
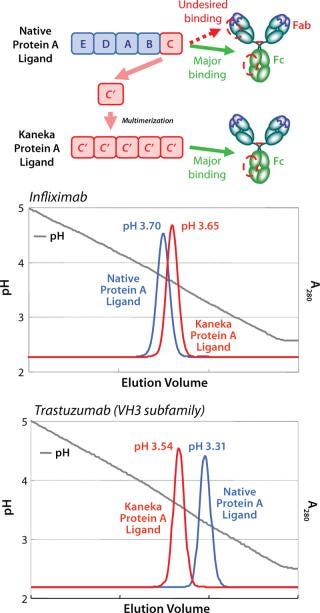
Figure 1: ()
Alkaline Stability of Kaneka Protein A Cellulose
CIP with sodium hydroxide is a robust cleaning and sanitization step for chromatography resins. Kaneka’s new ligand has excellent alkaline stability and can be used up to 300 cycles using 0.1M sodium hydroxide as CIP solution for 15 minutes of contact time with a limited loss of binding capacity (∼20%). In addition, 0.5M sodium hydroxide can be used up to 100 cycles (Figure 2). Yields in elution pools are stable, and leaching of protein A ligand is not increasing during cycle use. Because alkaline CIP can eliminate the usage of urea or guanidine-HCl, handling costs for dealing with these hazardous and expensive corrosive reagents are significantly reduced.
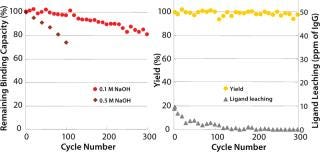
Figure 2: ()
Highly Cross-Linked Cellulose for Easy Scale-Up to Production
Kaneka’s cellulose base matrix has superior nonspecific binding characteristcs when compared with other matrices, polymers, or glass. Cellulose is biocompatible and has been used for many years in the plasmapheresis and dialysis fields. Kaneka protein A cellulose uses cellulose beads produced using innovative highly cross linking technology, which has allowed Kaneka to develop a resin that is easy to use, durable, and applicable to large-scale operations.
Figure 3 shows pressure/flow-rate characteristics and packing efficiency of the packed columns, from 4.4 cm to 45 cm i.d. and 20 cm bed height. A 10% glycerol solution for 45 cm I.D. column was used to simulate viscous cell culture feed stock, and it showed reasonable pressure increase.
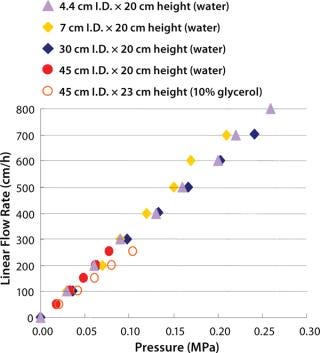
Figure 3: ()
Kaneka Protein A Cellulose can be packed using flow and axial compression packing methods from pilot to large scale without special equipment and techniques. The columns show excellent pressure flow characteristics with good column performance.
High Performance in Mab Production
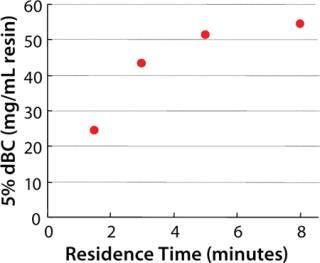
Dynamic binding capacity (dBC) is an important parameter when selecting a chromatography resin and is related to productivity and production cost. Kaneka Protein A Cellulose could achieve high productivity at residence times of 4 to 6 minutes (Figure 4). Moreover, a steep elution peak is observed using small elution volumes with mild elution pH, and both low- and high-titer feed stocks can be treated using this new Kaneka Protein A Cellulose (data not shown).
none;background-color: #f7f7f7;color:#000000;” >Figure 4: ()
Table 1: Test conditions for
Figure 3 — 1%(v/v) acetone sample injected at 1% of column volume and a 60-cm/h flow rate
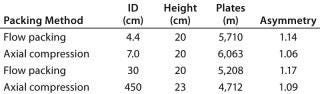
Table 1: Test conditions for Figure 3 — 1%(v/v) acetone sample injected at 1% of column volume and a 60-cm/h flow rate ()
In conclusion, Kaneka Protein A Cellulose demonstrates an overall high performance in the Mab capture step, making it an indispensable tool for your MAb purification platform.
About the Author
Author Details
Kazunobu Minakuchi is a senior researcher, Masahiro Funaki and Hiroaki Kawasaki are researchers, and Koji Iritani is head of the sales and development team in the Fine Chemicals Group (QOL Division) of Kaneka Corporation, 3-2-4 Nakanoshima, Kita-ku Osaka 530-8288, Japan; [email protected].
You May Also Like